Human Influenza A Virus Hemagglutinin Glycan Evolution Follows a Temporal Pattern to a Glycan Limit
- PMID: 30940704
- PMCID: PMC6445938
- DOI: 10.1128/mBio.00204-19
Human Influenza A Virus Hemagglutinin Glycan Evolution Follows a Temporal Pattern to a Glycan Limit
Abstract
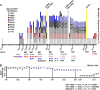
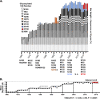
Human antibody-based immunity to influenza A virus is limited by antigenic drift resulting from amino acid substitutions in the hemagglutinin (HA) head domain. Glycan addition can cause large antigenic changes but is limited by fitness costs to viral replication. Here, we report that glycans are added to H1 and H3 HAs at discrete 5-to-7-year intervals, until they reach a functional glycan limit, after which glycans are swapped at approximately 2-fold-longer intervals. Consistent with this pattern, 2009 pandemic H1N1 added a glycan at residue N162 over the 2015-2016 season, an addition that required two epistatic HA head mutations for complete glycosylation. These strains rapidly replaced H1N1 strains globally, by 2017 dominating H3N2 and influenza B virus strains for the season. The pattern of glycan modulation that we outline should aid efforts for tracing the epidemic potential of evolving human IAV strains.IMPORTANCE Frequent mutation of its major antibody target, the glycoprotein hemagglutinin, ensures that the influenza virus is perennially both a rapidly emerging virus and a major threat to public health. One type of mutation escapes immunity by adding a glycan onto an area of hemagglutinin that many antibodies recognize. This study revealed that these glycan changes follow a simple temporal pattern. Every 5 to 7 years, hemagglutinin adds a new glycan, up to a limit. After this limit is reached, no net additions of glycans occur. Instead, glycans are swapped or lost at longer intervals. Eventually, a pandemic replaces the terminally glycosylated hemagglutinin with a minimally glycosylated one from the animal reservoir, restarting the cycle. This pattern suggests the following: (i) some hemagglutinins are evolved for this decades-long process, which is both defined by and limited by successive glycan addition; and (ii) hemagglutinin's antibody dominance and its capacity for mutations are highly adapted features that allow influenza to outpace our antibody-based immunity.
Keywords: glycosylation; hemagglutinin; immune evasion; influenza; viral evolution.
Figures




References
-
- WHO. 2003. Influenza fact sheet no. 211. http://www.who.int/mediacentre/factsheets/2003/fs211/en/. Accessed 21 June 2018.
-
- CDC. 2017. Vaccine effectiveness-how well does the flu vaccine work? https://www.cdc.gov/flu/about/qa/vaccineeffect.htm. Accessed 21 June 2018.
-
- Keil W, Geyer R, Dabrowski J, Dabrowski U, Niemann H, Stirm S, Klenk HD. 1985. Carbohydrates of influenza virus. Structural elucidation of the individual glycans of the FPV hemagglutinin by two-dimensional 1H n.m.r. and methylation analysis. EMBO J 4:2711–2720. doi: 10.1002/j.1460-2075.1985.tb03991.x. - DOI - PMC - PubMed
-
- Khatri K, Klein JA, White MR, Grant OC, Leymarie N, Woods RJ, Hartshorn KL, Zaia J. 2016. Integrated omics and computational glycobiology reveal structural basis for influenza A virus glycan microheterogeneity and host interactions. Mol Cell Proteomics 15:1895–1912. doi: 10.1074/mcp.M116.058016. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

