Tyrosine as a Mechanistic-Based Biomarker for Brain Glycogen Decrease and Supercompensation With Endurance Exercise in Rats: A Metabolomics Study of Plasma
- PMID: 30941004
- PMCID: PMC6433992
- DOI: 10.3389/fnins.2019.00200
Tyrosine as a Mechanistic-Based Biomarker for Brain Glycogen Decrease and Supercompensation With Endurance Exercise in Rats: A Metabolomics Study of Plasma
Abstract
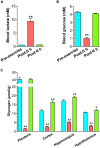
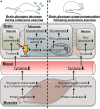
Brain glycogen, localized in astrocytes, produces lactate as an energy source and/or a signal factor to serve neuronal functions involved in memory formation and exercise endurance. In rodents, 4 weeks of chronic moderate exercise-enhancing endurance and cognition increases brain glycogen in the hippocampus and cortex, which is an adaption of brain metabolism achieved through exercise. Although this brain adaptation is likely induced due to the accumulation of acute endurance exercise-induced brain glycogen supercompensation, its molecular mechanisms and biomarkers are unidentified. Since noradrenaline synthesized from blood-borne tyrosine activates not only glycogenolysis but also glycogenesis in astrocytes, we hypothesized that blood tyrosine is a mechanistic-based biomarker of acute exercise-induced brain glycogen supercompensation. To test this hypothesis, we used a rat model of endurance exercise, a microwave irradiation for accurate detection of glycogen in the brain (the cortex, hippocampus, and hypothalamus), and capillary electrophoresis mass spectrometry-based metabolomics to observe the comprehensive metabolic profile of the blood. Endurance exercise induced fatigue factors such as a decrease in blood glucose, an increase in blood lactate, and the depletion of muscle glycogen, but those parameters recovered to basal levels within 6 h after exercise. Brain glycogen decreased during endurance exercise and showed supercompensation within 6 h after exercise. Metabolomics detected 186 metabolites in the plasma, and 110 metabolites changed significantly during and following exhaustive exercise. Brain glycogen levels correlated negatively with plasma glycogenic amino acids (serine, proline, threonine, glutamate, methionine, tyrosine, and tryptophan) (r < -0.9). This is the first study to produce a broad picture of plasma metabolite changes due to endurance exercise-induced brain glycogen supercompensation. Our findings suggest that plasma glycogenic amino acids are sensitive indicators of brain glycogen levels in endurance exercise. In particular, plasma tyrosine as a precursor of brain noradrenaline might be a valuable mechanistic-based biomarker to predict brain glycogen dynamics in endurance exercise.
Keywords: brain glycogen; endurance exercise; metabolomics; plasma biomarker; supercompensation.
Figures





Similar articles
-
Endurance and Brain Glycogen: A Clue Toward Understanding Central Fatigue.Adv Neurobiol. 2019;23:331-346. doi: 10.1007/978-3-030-27480-1_11. Adv Neurobiol. 2019. PMID: 31667814
-
Astrocytic glycogen-derived lactate fuels the brain during exhaustive exercise to maintain endurance capacity.Proc Natl Acad Sci U S A. 2017 Jun 13;114(24):6358-6363. doi: 10.1073/pnas.1702739114. Epub 2017 May 17. Proc Natl Acad Sci U S A. 2017. PMID: 28515312 Free PMC article.
-
Brain glycogen supercompensation following exhaustive exercise.J Physiol. 2012 Feb 1;590(3):607-16. doi: 10.1113/jphysiol.2011.217919. Epub 2011 Nov 7. J Physiol. 2012. PMID: 22063629 Free PMC article.
-
The regulation of carbohydrate and fat metabolism during and after exercise.Front Biosci. 1998 Sep 15;3:D1011-27. doi: 10.2741/a342. Front Biosci. 1998. PMID: 9740552 Review.
-
Carbohydrate-loading and exercise performance. An update.Sports Med. 1997 Aug;24(2):73-81. doi: 10.2165/00007256-199724020-00001. Sports Med. 1997. PMID: 9291549 Review.
Cited by
-
Metformin Protects Rat Skeletal Muscle from Physical Exercise-Induced Injury.Biomedicines. 2023 Aug 22;11(9):2334. doi: 10.3390/biomedicines11092334. Biomedicines. 2023. PMID: 37760776 Free PMC article.
-
Comparison of intrinsic exercise capacity and response to acute exercise in ICR (Institute of Cancer Research) mice derived from three different lineages.Lab Anim Res. 2021 Aug 4;37(1):21. doi: 10.1186/s42826-021-00094-0. Lab Anim Res. 2021. PMID: 34348800 Free PMC article.
-
The metabolic recovery of marathon runners: an untargeted 1H-NMR metabolomics perspective.Front Physiol. 2023 May 4;14:1117687. doi: 10.3389/fphys.2023.1117687. eCollection 2023. Front Physiol. 2023. PMID: 37215177 Free PMC article.
-
Precision nutrition in sports science: an opinion on omics-based personalization and athletic outcomes.Front Nutr. 2025 Jun 6;12:1611440. doi: 10.3389/fnut.2025.1611440. eCollection 2025. Front Nutr. 2025. PMID: 40547365 Free PMC article. No abstract available.
-
Metabolomics in Exercise and Sports: A Systematic Review.Sports Med. 2022 Mar;52(3):547-583. doi: 10.1007/s40279-021-01582-y. Epub 2021 Oct 30. Sports Med. 2022. PMID: 34716906
References
LinkOut - more resources
Full Text Sources

