Emerging Roles of the TRIM E3 Ubiquitin Ligases MID1 and MID2 in Cytokinesis
- PMID: 30941058
- PMCID: PMC6433704
- DOI: 10.3389/fphys.2019.00274
Emerging Roles of the TRIM E3 Ubiquitin Ligases MID1 and MID2 in Cytokinesis
Abstract
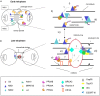
Ubiquitination is a post-translational modification that consists of ubiquitin attachment to target proteins through sequential steps catalysed by activating (E1), conjugating (E2), and ligase (E3) enzymes. Protein ubiquitination is crucial for the regulation of many cellular processes not only by promoting proteasomal degradation of substrates but also re-localisation of cellular factors and modulation of protein activity. Great importance in orchestrating ubiquitination relies on E3 ligases as these proteins recognise the substrate that needs to be modified at the right time and place. Here we focus on two members of the TRIpartite Motif (TRIM) family of RING E3 ligases, MID1, and MID2. We discuss the recent findings on these developmental disease-related proteins analysing the link between their activity on essential factors and the regulation of cytokinesis highlighting the possible consequence of alteration of this process in pathological conditions.
Keywords: MID1; MID2; TRIM E3 ligase; X-linked Opitz syndrome; cytokinesis; ubiquitination.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

