Treating Disorders of Consciousness With Apomorphine: Protocol for a Double-Blind Randomized Controlled Trial Using Multimodal Assessments
- PMID: 30941094
- PMCID: PMC6433751
- DOI: 10.3389/fneur.2019.00248
Treating Disorders of Consciousness With Apomorphine: Protocol for a Double-Blind Randomized Controlled Trial Using Multimodal Assessments
Abstract
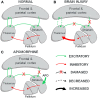
Background: There are few available therapeutic options to promote recovery among patients with chronic disorders of consciousness (DOC). Among pharmacological treatments, apomorphine, a dopamine agonist, has exhibited promising behavioral effects and safety of use in small-sample pilot studies. The true efficacy of the drug and its neural mechanism are still unclear. Apomorphine may act through a modulation of the anterior forebrain mesocircuit, but neuroimaging and neurophysiological investigations to test this hypothesis are scarce. This clinical trial aims to (1) assess the treatment effect of subcutaneous apomorphine infusions in patients with DOC, (2) better identify the phenotype of responders to treatment, (3) evaluate tolerance and side effects in this population, and (4) examine the neural networks underlying its modulating action on consciousness. Methods/Design: This study is a prospective double-blind randomized parallel placebo-controlled trial. Forty-eight patients diagnosed with DOC will be randomized to receive a 30-day regimen of either apomorphine hydrochloride or placebo subcutaneous infusions. Patients will be monitored at baseline 30 days before initiation of therapy, during treatment and for 30 days after treatment washout, using standardized behavioral scales (Coma Recovery Scale-Revised, Nociception Coma Scale-Revised), neurophysiological measures (electroencephalography, body temperature, actigraphy) and brain imaging (magnetic resonance imaging, positron emission tomography). Behavioral follow-up will be performed up to 2 years using structured phone interviews. Analyses will look for changes in behavioral status, circadian rhythmicity, brain metabolism, and functional connectivity at the individual level (comparing before and after treatment) and at the group level (comparing apomorphine and placebo arms, and comparing responder and non-responder groups). Discussion: This study investigates the use of apomorphine for the recovery of consciousness in the first randomized placebo-controlled double-blind trial using multimodal assessments. The results will contribute to define the role of dopamine agonists for the treatment of these challenging conditions and identify the neural correlates to their action. Results will bring objective evidence to further assess the modulation of the anterior forebrain mesocircuit by pharmacological agents, which may open new therapeutic perspectives. Clinical Trial Registration: EudraCT n°2018-003144-23; Clinicaltrials.gov n°NCT03623828 (https://clinicaltrials.gov/ct2/show/NCT03623828).
Keywords: apomorphine; clinical trial; disorders of consciousness; dopamine; mesocircuit; minimally conscious state; protocol; unresponsive wakefulness syndrome.
Figures

References
Publication types
Associated data
LinkOut - more resources
Full Text Sources
Medical

