There Is Selective Increase in Pro-thrombotic Circulating Extracellular Vesicles in Acute Ischemic Stroke and Transient Ischemic Attack: A Study of Patients From the Middle East and Southeast Asia
- PMID: 30941096
- PMCID: PMC6434679
- DOI: 10.3389/fneur.2019.00251
There Is Selective Increase in Pro-thrombotic Circulating Extracellular Vesicles in Acute Ischemic Stroke and Transient Ischemic Attack: A Study of Patients From the Middle East and Southeast Asia
Abstract
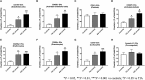
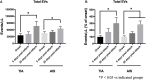
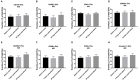
Stroke attacks were found to be present at a younger age in patients from Southeast Asia (SE) and the Middle East (ME) resident in the state of Qatar. Extracellular vesicles (EVs), which are small membrane vesicles with pro-thrombotic properties, may contribute to the high risk of stroke in this population. Thus, total and cell-specific medium size EVs were counted by flow cytometry in platelet-free plasma from healthy volunteers and patients with transient ischemic attacks (TIA) and acute ischemic stroke (AIS) from SE and ME. Acutely, within 48 h of attacks, there was an increase in total endothelial EVs in TIA (6.73 ± 1.77; P = 0.0156; n = 21) and AIS (11.23 ± 1.95; P = 0.0007; n = 66) patients compared to controls (2.04 ± 0.78; n = 24). Similar increases were also evident in EVs originating from platelets, erythrocytes, granulocytes, and leukocytes. Compared to controls, there was also an increase in EVs derived from activated endothelial cells, platelets, granulocytes, leukocytes, and pro-coagulant EVs (Annexin V+) at 5 and 30-days following the acute events, while a decrease was observed in erythrocyte-derived EVs. This is the first study characterizing EVs in TIA and AIS patients from ME and SE showing an increase in EVs associated with endothelial and platelet cell activation, which may contribute to the elevated risk of stroke at a younger age in this population.
Keywords: acute ischemic stroke (AIS); biomarkers; extracellular vesicles (EVs); thrombosis; transient ischemic attacks (TIA).
Figures



References
LinkOut - more resources
Full Text Sources

