The Emerging Role of Complement Proteins as a Target for Therapy of IgA Nephropathy
- PMID: 30941137
- PMCID: PMC6433978
- DOI: 10.3389/fimmu.2019.00504
The Emerging Role of Complement Proteins as a Target for Therapy of IgA Nephropathy
Abstract
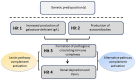
IgA nephropathy (IgAN) is the most common form of primary glomerulonephritis worldwide and a common cause of end-stage renal disease. Evaluation of a kidney biopsy is necessary for diagnosis, with routine immunofluorescence microscopy revealing dominant or co-dominant IgA immunodeposits usually with complement C3 and sometimes IgG and/or IgM. IgA nephropathy reduces life expectancy by more than 10 years and leads to kidney failure in 20-40% of patients within 20 years of diagnosis. There is accumulating clinical, genetic, and biochemical evidence that complement plays an important role in the pathogenesis of IgA nephropathy. The presence of C3 differentiates the diagnosis of IgA nephropathy from the subclinical deposition of glomerular IgA. Markers for the activation of the alternative and mannan-binding lectin (MBL) pathways in renal-biopsy specimens are associated with disease activity and portend a worse renal outcome. Complement proteins in the circulation have also been evaluated in IgA nephropathy and found to be of prognostic value. Recently, genetic studies have identified IgA nephropathy-associated loci. Within these loci are genes encoding products involved in complement regulation and interaction with immune complexes. Put together, these data identify the complement cascade as a rational treatment target for this chronic kidney disease. Recent case reports on the successful use of humanized anti-C5 monoclonal antibody eculizumab are consistent with this hypothesis, but a better understanding of the role of complement in IgA nephropathy is needed to guide future therapeutic interventions.
Keywords: IgA nephropathy; IgAN; IgAN pathogenesis; IgAN treatment; alternative complement pathway; complement; mannan binding lectin complement pathway.
Figures

References
-
- Berger J, Hinglais N. Intercapillary deposits of IgA-IgG. J Urol Nephrol. (1968) 74:694–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

