Real World Experience of Chronic Hepatitis C Retreatment with Genotype Specific Regimens in Nonresponders to Previous Interferon-Free Therapy
- PMID: 30941326
- PMCID: PMC6420981
- DOI: 10.1155/2019/4029541
Real World Experience of Chronic Hepatitis C Retreatment with Genotype Specific Regimens in Nonresponders to Previous Interferon-Free Therapy
Abstract
Background and aim: The development of interferon- (IFN-) free regimens substantially improved efficacy of treatment for HCV, but despite excellent effectiveness the failures still occur. The aim of our study was to evaluate the efficacy of retreatment with genotype specific direct acting antivirals- (DAA-) based regimens in nonresponders to previous IFN-free therapy.
Materials and methods: Analysed population consisted of 31 nonresponders to IFN-free regimen, which received second IFN-free rescue therapy, selected from 6228 patients included in a national database EpiTer-2.
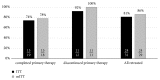
Results: Age and gender distribution were similar, whereas proportion of genotype 1b was slightly higher and genotype 4 lower in the whole population compared to studied one. Patients included in the study demonstrated much more advanced fibrosis. Primary therapy was discontinued in 12 patients, which were recognized as failures due to nonvirologic reason, whereas virologic reason of therapeutic failure was recognized in 19 patients which completed therapy. Overall sustained virologic response (SVR) rate was 81% and 86% in intent-to-treat (ITT) and modified ITT analysis, respectively (74% and 78% in virologic failures, 92% and 100% in nonvirologic failures). Resistance-associated substitutions (RAS) testing was carried out in 8 patients from the group of completed primary therapy and three of them had potential risk for failure of rescue therapy due to NS5A association, while two of them achieved SVR.
Conclusions: We demonstrated moderate effectiveness of genotype specific rescue therapy in failures due to virologic reason and high in those who discontinued primary therapy. Therefore rescue therapy with genotype specific regimens should be considered always if more potent regimens are not available.
Figures
References
-
- Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. The Lancet Gastroenterology & Hepatology. 2017;2:161–176. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical