Distinct Profiles on Subjective and Objective Adherence Measures in Patients Prescribed Antidepressants
- PMID: 30941607
- PMCID: PMC6483946
- DOI: 10.1007/s40265-019-01107-y
Distinct Profiles on Subjective and Objective Adherence Measures in Patients Prescribed Antidepressants
Abstract
Objective: A recurrent observation is that associations between self-reported and objective medication adherence measures are often weak to moderate. Our aim was therefore to identify patients with different profiles on self-reported and objective adherence measures.
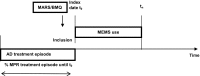
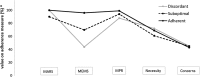
Study design and setting: This was an observational study of 221 community pharmacy patients who were dispensed antidepressants. Adherence profiles were estimated with Latent Profile Analysis (LPA) using data on self-reported adherence (Medication Adherence Rating Scale) complemented with data on medication beliefs (perceived necessity and concerns measured with the Beliefs about Medicines Questionnaire) and data from objective adherence measures (electronic monitoring of medication taking and the Medication Possession Ratio calculated from pharmacy dispensing data).
Results: 'Goodness-of-fit' statistics indicated the presence of three classes: "concordantly high adherent" (83%, high adherence on all measures), "concordantly suboptimal adherent" (11%, low adherence on all measures), and "discordant" (6%, high self-reported adherence but lower adherence on objective measures).
Conclusion: Most patients had concordant outcomes on self-reported and objective measures of adherence. A small discordant class had high self-reported but low objective adherence. LPA will enable sensitivity analyses in future studies, for example excluding patients from the discordant class.
Conflict of interest statement
Conflict of interest
The authors report no other conflicts of interest.
Ethical standards
All procedures performed in this study were in accordance with the ethical standards of the medical ethics committee of the University Medical Center of Utrecht, which approved the study, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all individual participants who were included in the study.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

