Cryptochrome-mediated hypocotyl phototropism was regulated antagonistically by gibberellic acid and sucrose in Arabidopsis
- PMID: 30941890
- PMCID: PMC7318699
- DOI: 10.1111/jipb.12813
Cryptochrome-mediated hypocotyl phototropism was regulated antagonistically by gibberellic acid and sucrose in Arabidopsis
Abstract
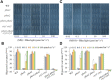
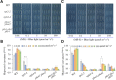
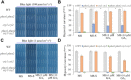
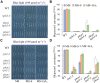
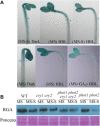
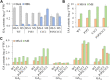
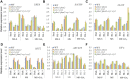
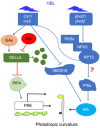
Both phototropins (phot1 and phot2) and cryptochromes (cry1 and cry2) were proven as the Arabidopsis thaliana blue light receptors. Phototropins predominately function in photomovement, and cryptochromes play a role in photomorphogenesis. Although cryptochromes have been proposed to serve as positive modulators of phototropic responses, the underlying mechanism remains unknown. Here, we report that depleting sucrose from the medium or adding gibberellic acids (GAs) can partially restore the defects in phototropic curvature of the phot1 phot2 double mutants under high-intensity blue light; this restoration does not occur in phot1 phot2 cry1 cry2 quadruple mutants and nph3 (nonphototropic hypocotyl 3) mutants which were impaired phototropic response in sucrose-containing medium. These results indicate that GAs and sucrose antagonistically regulate hypocotyl phototropism in a cryptochromes dependent manner, but it showed a crosstalk with phototropin signaling on NPH3. Furthermore, cryptochromes activation by blue light inhibit GAs synthesis, thus stabilizing DELLAs to block hypocotyl growth, which result in the higher GAs content in the shade side than the lit side of hypocotyl to support the asymmetric growth of hypocotyl. Through modulation of the abundance of DELLAs by sucrose depletion or added GAs, it revealed that cryptochromes have a function in mediating phototropic curvature.
© 2019 The Authors. Journal of Integrative Plant Biology Published by John Wiley & Sons Australia, Ltd on behalf of Institute of Botany, Chinese Academy of Sciences.
Figures
References
-
- Ahmad M, Jarillo JA, Smirnova O, Cashmore AR (1998) Cryptochrome blue‐light photoreceptors of Arabidopsis implicated in phototropism. Nature 392: 720–723 - PubMed
-
- Briggs WR, Tseng TS, Cho HY, Swartz TE, Sullivan S, Bogomolni RA, Kaiserli E, Christie JM (2007) Phototropins and their LOV domains: Versatile plant blue‐light receptors. J Integr Plant Biol 49: 4–10
-
- Chen ML, Fu XM, Liu JQ, Ye TT, Hou SY, Huang YQ, Yuan BF, Wu Y, Feng YQ (2012) Highly sensitive and quantitative profiling of acidic phytohormones using derivatization approach coupled with nano‐LC‐ESI‐Q‐TOF‐MS analysis. J Chromatogr B 905: 67 –74 - PubMed
-
- Christie JM, Murphy AS (2013) Shoot phototropism in higher plants: New light through old concepts. Am J Bot 100: 35–46 - PubMed
-
- Christie JM, Reymond P, Powell GK, Bernasconi P, Raibekas AA, Liscum E, Briggs WR (1998) Arabidopsis NP H1: A flavoprotein with the properties of a photoreceptor for phototropism. Science 282: 1698–1701 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

