The Injectisome, a Complex Nanomachine for Protein Injection into Mammalian Cells
- PMID: 30942149
- PMCID: PMC6450406
- DOI: 10.1128/ecosalplus.ESP-0039-2018
The Injectisome, a Complex Nanomachine for Protein Injection into Mammalian Cells
Abstract
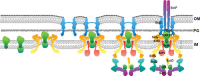
Type III protein secretion systems (T3SSs), or injectisomes, are multiprotein nanomachines present in many Gram-negative bacteria that have a sustained long-standing close relationship with a eukaryotic host. These secretion systems have evolved to modulate host cellular functions through the activity of the effector proteins they deliver. To reach their destination, T3SS effectors must cross the multibarrier bacterial envelope and the eukaryotic cell membrane. Passage through the bacterial envelope is mediated by the needle complex, a central component of T3SSs that expands both the inner and outer membranes of Gram-negative bacteria. A set of T3SS secreted proteins, known as translocators, form a channel in the eukaryotic plasma membrane through which the effector proteins are delivered to reach the host cell cytosol. While the effector proteins are tailored to the specific lifestyle of the bacterium that encodes them, the injectisome is conserved among the different T3SSs. The central role of T3SSs in pathogenesis and their high degree of conservation make them a desirable target for the development of antimicrobial therapies against several important bacterial pathogens.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

