Weight and Height in Children and Adolescents with Attention-Deficit/Hyperactivity Disorder: A Longitudinal Database Study Assessing the Impact of Guanfacine, Stimulants, and No Pharmacotherapy
- PMID: 30942617
- PMCID: PMC6534094
- DOI: 10.1089/cap.2018.0132
Weight and Height in Children and Adolescents with Attention-Deficit/Hyperactivity Disorder: A Longitudinal Database Study Assessing the Impact of Guanfacine, Stimulants, and No Pharmacotherapy
Abstract
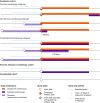
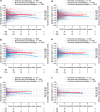
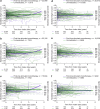
Objectives: To assess the impact of long-term pharmacotherapy with guanfacine immediate- or extended-release (GXR), administered alone or as an adjunctive to a stimulant, on weight and height in children and adolescents with attention-deficit/hyperactivity disorder (ADHD). Methods: Data were extracted from U.S. Department of Defense medical records for patients 4-17 years of age at index date (initiation of any study medication following a year without ADHD medications, or diagnosis if unmedicated) with weight/height measurements for the analysis period (January 2009-June 2013) and the previous year (baseline). Longitudinal weight and height z-scores were analyzed using multivariable regression in three cohorts: guanfacine (initial period of guanfacine exposure), first-line stimulant monotherapy (initial period of exposure), and unmedicated. Guanfacine cohort subgroups were based on previous/concurrent stimulant exposure. Results: The weight analyses included 47,910 patients (66.8% male) and the height analyses 41,248 (67.2% male). Mean initial exposure in the weight analyses was 237 days (standard deviation [SD] = 258, median = 142) for guanfacine and 257 days (SD = 284, median = 151) for first-line stimulant monotherapy, and was similar in the height analyses. Modeling indicated that guanfacine monotherapy was not associated with clinically meaningful deviations from normal z-score trajectories for weight (first-line, n = 943; nonfirst-line, n = 796) or height (first-line, n = 741; nonfirst-line, n = 644). In patients receiving guanfacine adjunctive to a stimulant, modeled weight (n = 1657) and height (n = 1343) z-scores followed declining trajectories. In this subgroup, mean standardized weight/height had decreased during previous stimulant monotherapy. For first-line stimulant monotherapy, modeled weight (n = 32,999) and height (n = 28,470) z-scores followed declining trajectories during year 1. In the unmedicated cohort, modeled weight (n = 11,515) and height (n = 10,050) z-scores were stable. Conclusions: Guanfacine monotherapy (first-line or nonfirst-line) was not associated with marked deviations from normal growth in this modeling study of children and adolescents with ADHD. In contrast, growth trajectories followed an initially declining course with stimulants, whether given alone or with adjunctive guanfacine.
Keywords: attention-deficit/hyperactivity disorder; guanfacine; height; stimulant; weight.
Conflict of interest statement
This study was funded by Shire Development LLC, now part of Takeda. Shire, now part of Takeda, develops and markets medications for ADHD. Evidera received funding from Shire Development LLC for designing and conducting this study and analyzing and reporting the results. Oxford PharmaGenesis, Oxford, United Kingdom received funding from Shire International GmbH for writing and editing support. Under the direction of the authors, writing assistance was provided by Dr. Heather Lang and Dr. Matt Cottingham, employees of Oxford PharmaGenesis; editorial assistance in formatting, proofreading, copy editing, and fact checking was also provided by Oxford PharmaGenesis. All authors had full access to the study report and results, and had final responsibility for the decision to submit the article for publication. All authors contributed to the preparation of this article and to interpretation of the results. G.S. designed the study. G.S. and B.M. performed the statistical analyses. W.M.S. is an employee of Shire, now part of Takeda. B.M. and M.R. are employees of Evidera. G.S. is a former employee of Evidera. B.L.F. is a military service member. This work was prepared as part of his official duties.
The following authors have received compensation for serving as consultants or speakers for, or they or the institutions they work for have received research support or royalties from, the companies or organizations indicated: T.B. (Actelion, Hexal Pharma, Lilly, Lundbeck, Medice, Neurim Pharmaceuticals, Novartis, Shire, and Vifor Pharma), P.A.G. (Lilly and Shire), D.R.C. (Eli Lilly, Janssen-Cilag, Medice, Novartis, Oxford University Press, and Shire).
Research data derived from an approved Naval Medical Center, Portsmouth, VA IRB protocol (NMCP.2013.0053). The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the United States Government. Copyright Notice: CAPT B.L.F. is a military service member. This work was prepared as part of his official duties. Title 17 U.S.C. 105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. 101 defines a United States Government work as a work prepared by a military service member or employee of the United States Government as part of that person's official duties.
The corresponding author can be contacted for further details about the study and the data.
Figures

Similar articles
-
Cost effectiveness of guanfacine extended release as an adjunctive therapy to a stimulant compared with stimulant monotherapy for the treatment of attention-deficit hyperactivity disorder in children and adolescents.Pharmacoeconomics. 2012 Aug 1;30(8):e1-15. doi: 10.2165/11632920-000000000-00000. Pharmacoeconomics. 2012. PMID: 22788263 Free PMC article. Clinical Trial.
-
Guanfacine extended release as adjunctive therapy to psychostimulants in children and adolescents with attention-deficit/hyperactivity disorder.Adv Ther. 2012 May;29(5):385-400. doi: 10.1007/s12325-012-0020-1. Epub 2012 May 18. Adv Ther. 2012. PMID: 22610723 Review.
-
Evaluation of the current data on guanfacine extended release for the treatment of ADHD in children and adolescents.Expert Opin Pharmacother. 2020 Mar;21(4):417-426. doi: 10.1080/14656566.2019.1706480. Epub 2020 Jan 23. Expert Opin Pharmacother. 2020. PMID: 31971448 Review.
-
The impact of adjunctive guanfacine extended release on stimulant adherence in children/adolescents with attention-deficit/hyperactivity disorder.J Comp Eff Res. 2017 Mar;6(2):109-125. doi: 10.2217/cer-2016-0039. Epub 2017 Jan 25. J Comp Eff Res. 2017. PMID: 28118752
-
Is adjunctive pharmacotherapy in attention-deficit/hyperactivity disorder cost-effective in Canada: a cost-effectiveness assessment of guanfacine extended-release as an adjunctive therapy to a long-acting stimulant for the treatment of ADHD.BMC Psychiatry. 2016 Jan 16;16:11. doi: 10.1186/s12888-016-0708-x. BMC Psychiatry. 2016. PMID: 26774811 Free PMC article.
Cited by
-
Clinically Significant Drug-Drug Interactions with Agents for Attention-Deficit/Hyperactivity Disorder.CNS Drugs. 2019 Dec;33(12):1201-1222. doi: 10.1007/s40263-019-00683-7. CNS Drugs. 2019. PMID: 31776871 Review.
-
Insights from the largest diverse ancestry sex-specific disease map for genetically predicted height.NPJ Genom Med. 2025 Feb 27;10(1):14. doi: 10.1038/s41525-025-00464-w. NPJ Genom Med. 2025. PMID: 40016231 Free PMC article.
References
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision (DSM-IV-TR). Washington, DC: American Psychiatric Association; 2000
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed., (DSM-5). Washington, D.C: American Psychiatric Association; 2013
-
- Biederman J, Melmed RD, Patel A, McBurnett K, Donahue J, Lyne A: Long-term, open-label extension study of guanfacine extended release in children and adolescents with ADHD. CNS Spectr 13:1047–1055, 2008a - PubMed
-
- Biederman J, Melmed RD, Patel A, McBurnett K, Konow J, Lyne A, Scherer N; SPD503 Study Group: A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics 121:e73–e84, 2008b - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
