A dimensional warehouse for integrating operational data from clinical trials
- PMID: 30942863
- PMCID: PMC6446529
- DOI: 10.1093/database/baz039
A dimensional warehouse for integrating operational data from clinical trials
Abstract
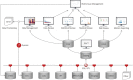
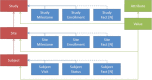
Timely, consistent and integrated access to clinical trial data remains one of the pharmaceutical industry's most pressing needs. As part of a comprehensive clinical data repository, we have developed a data warehouse that can integrate operational data from any source, conform it to a canonical data model and make it accessible to study teams in a timely, secure and contextualized manner to support operational oversight, proactive risk management and other analytic and reporting needs. Our solution consists of a dimensional relational data warehouse, a set of extraction, transformation and loading processes to coordinate data ingestion and mapping, a generalizable metrics engine to enable the computation of operational metrics and key performance, quality and risk indicators and a set of graphical user interfaces to facilitate configuration, management and administration. When combined with the appropriate data visualization tools, the warehouse enables convenient access to raw operational data and derived metrics to help track study conduct and performance, identify and mitigate risks, monitor and improve operational processes, manage resource allocation, strengthen investigator and sponsor relationships and other purposes.
© The Author(s) 2019. Published by Oxford University Press.
Figures







References
-
- Bose A. and Das S. (2012) Trial analytics: a tool for clinical trial management. Acta Pol. Pharm., 69, 523–533. - PubMed
-
- Kimball R. and Ross M. (2002) The Data Warehouse Toolkit: The Complete Guide to Dimensional Modeling, 2nd edn. Wiley Computer Publishing, New York.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

