A Radical Approach to Anionic Chemistry: Synthesis of Ketones, Alcohols, and Amines
- PMID: 30943023
- PMCID: PMC7004484
- DOI: 10.1021/jacs.9b02238
A Radical Approach to Anionic Chemistry: Synthesis of Ketones, Alcohols, and Amines
Abstract
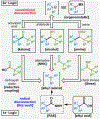
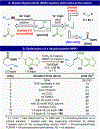
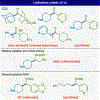
Historically accessed through two-electron, anionic chemistry, ketones, alcohols, and amines are of foundational importance to the practice of organic synthesis. After placing this work in proper historical context, this Article reports the development, full scope, and a mechanistic picture for a strikingly different way of forging such functional groups. Thus, carboxylic acids, once converted to redox-active esters (RAEs), can be utilized as formally nucleophilic coupling partners with other carboxylic derivatives (to produce ketones), imines (to produce benzylic amines), or aldehydes (to produce alcohols). The reactions are uniformly mild, operationally simple, and, in the case of ketone synthesis, broad in scope (including several applications to the simplification of synthetic problems and to parallel synthesis). Finally, an extensive mechanistic study of the ketone synthesis is performed to trace the elementary steps of the catalytic cycle and provide the end-user with a clear and understandable rationale for the selectivity, role of additives, and underlying driving forces involved.
Figures





References
-
- Kagan HB. Victor Grignard and Paul Sabatier: Two Showcase Laureates of the Nobel Prize for Chemistry. Angew. Chem. Int. Ed 2012, 51, 7376–7382. - PubMed
-
- Seyferth D. The Grignard Reagents. Organometallics 2009, 28, 1598–1605 and references therein.
-
- For reviews and selected examples, see: (a) Liu RY; Zhou Y; Yang Y; Buchwald SL. Enantioselective Allylation Using Allene, a Petroleum Cracking Byproduct. J. Am. Chem. Soc 2019, 141, 2251–2256. - PMC - PubMed
- Yang Y; Perry IB; Buchwald SL. Copper-Catalyzed Enantioselective Addition of Styrene-Derived Nucleophiles to Imines Enabled by Ligand-Controlled Chemoselective Hydrocupration. J. Am. Chem. Soc 2016, 138, 9787–9790. - PMC - PubMed
- Wang Y-M; Bruno NC; Placeres ÁL; Zhu S; Buchwald SL. Enantioselective Synthesis of Carbo- and Heterocycles through a CuH-Catalyzed Hydroalkylation Approach. J. Am. Chem. Soc 2015, 137, 10524–10527. - PMC - PubMed
- Zhou Y; Bandar JS; Buchwald SL. Enantioselective CuH-Catalyzed Hydroacylation Employing Unsaturated Carboxylic Acids as Aldehyde Surrogates. J. Am. Chem. Soc 2017, 139, 8126–8129. - PMC - PubMed
- Obora Y; Hatanaka S; Ishii Y. Iridium-Catalyzed Coupling Reaction of Primary Alcohols with 1-Aryl-1-propynes Leading to Secondary Homoallylic Alcohols. Org. Lett 2009, 11, 3510–3513. - PubMed
- Bower JF; Kim IS; Patman RL; Krische MJ. Catalytic Carbonyl Addition through Transfer Hydrogenation: A Departure from Preformed Organometallic Reagents. Angew. Chem. Int. Ed 2008, 48, 34–46. - PMC - PubMed
- Kokubo K; Miura M; Nomura M. Rhodium-Catalyzed Reaction of Benzoic Anhydride with Styrene under Molecular Hydrogen. Organometallics 1995, 14, 4521–4524.
- Kim SW; Zhang W; Krische MJ. Catalytic Enantioselective Carbonyl Allylation and Propargylation via Alcohol-Mediated Hydrogen Transfer: Merging the Chemistry of Grignard and Sabatier. Acc. Chem. Res 2017, 50, 2371–2380. - PMC - PubMed
- Nguyen KD; Park BY; Luong T; Sato H; Garza VJ; Krische MJ. Metal-catalyzed reductive coupling of olefin-derived nucleophiles: Reinventing carbonyl addition. Science 2016, 354, aah5133. - PMC - PubMed
- Deutsch C; Krause N; Lipshutz BH. CuH-Catalyzed Reactions. Chem. Rev 2008, 108, 2916–2927. - PubMed
- Mohr J; Oestreich M. Balancing C=C Functionalization and C=O Reduction in Cu−H Catalysis. Angew. Chem. Int. Ed 2016, 55, 12148–12149. - PubMed
-
- Crossley SWM; Obradors C; Martinez RM; Shenvi RA. Mn-, Fe-, and Co-Catalyzed Radical Hydrofunctionalizations of Olefins. Chemical Reviews 2016, 116, 8912–9000. - PMC - PubMed
- Green SA; Crossley SWM; Matos JLM; Vásquez-Céspedes S; Shevick SL; Shenvi RA. The High Chemofidelity of Metal-Catalyzed Hydrogen Atom Transfer. Acc. Chem. Res 2018, 51 (11), 2628–2640. - PMC - PubMed
- Lo JC; Kim D; Pan C-M; Edwards JT; Yabe Y; Gui J; Qin T; Gutiérrez S; Giacoboni J; Smith MW; Holland PL; Baran PS. Fe-Catalyzed C–C Bond Construction from Olefins via Radicals. J. Am. Chem. Soc 2017, 139, 2484–2503. - PMC - PubMed
- Shevick SL; Obradors C; Shenvi RA. Mechanistic Interrogation of Co/Ni-Dual Catalyzed Hydroarylation. J. Am. Chem. Soc 2018, 140, 12056–12068. - PMC - PubMed
-
- Gooßen LJ; Rodríguez N; Gooßen K. Carboxylic Acids as Substrates in Homogeneous Catalysis. Angew. Chem., Int. Ed 2008, 47, 3100–3120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

