Safety and Efficacy of a Five-Fraction Stereotactic Body Radiotherapy Schedule for Centrally Located Non-Small-Cell Lung Cancer: NRG Oncology/RTOG 0813 Trial
- PMID: 30943123
- PMCID: PMC6524984
- DOI: 10.1200/JCO.18.00622
Safety and Efficacy of a Five-Fraction Stereotactic Body Radiotherapy Schedule for Centrally Located Non-Small-Cell Lung Cancer: NRG Oncology/RTOG 0813 Trial
Abstract
Purpose: Patients with centrally located early-stage non-small-cell lung cancer (NSCLC) are at a higher risk of toxicity from high-dose ablative radiotherapy. NRG Oncology/RTOG 0813 was a phase I/II study designed to determine the maximum tolerated dose (MTD), efficacy, and toxicity of stereotactic body radiotherapy (SBRT) for centrally located NSCLC.
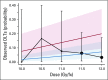
Materials and methods: Medically inoperable patients with biopsy-proven, positron emission tomography-staged T1 to 2 (≤ 5 cm) N0M0 centrally located NSCLC were accrued into a dose-escalating, five-fraction SBRT schedule that ranged from 10 to 12 Gy/fraction (fx) delivered over 1.5 to 2 weeks. Dose-limiting toxicity (DLT) was defined as any treatment-related grade 3 or worse predefined toxicity that occurred within the first year. MTD was defined as the SBRT dose at which the probability of DLT was closest to 20% without exceeding it.
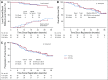
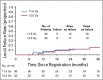
Results: One hundred twenty patients were accrued between February 2009 and September 2013. Patients were elderly, there were slightly more females, and the majority had a performance status of 0 to 1. Most cancers were T1 (65%) and squamous cell (45%). Organs closest to planning target volume/most at risk were the main bronchus and large vessels. Median follow-up was 37.9 months. Five patients experienced DLTs; MTD was 12.0 Gy/fx, which had a probability of a DLT of 7.2% (95% CI, 2.8% to 14.5%). Two-year rates for the 71 evaluable patients in the 11.5 and 12.0 Gy/fx cohorts were local control, 89.4% (90% CI, 81.6% to 97.4%) and 87.9% (90% CI, 78.8% to 97.0%); overall survival, 67.9% (95% CI, 50.4% to 80.3%) and 72.7% (95% CI, 54.1% to 84.8%); and progression-free survival, 52.2% (95% CI, 35.3% to 66.6%) and 54.5% (95% CI, 36.3% to 69.6%), respectively.
Conclusion: The MTD for this study was 12.0 Gy/fx; it was associated with 7.2% DLTs and high rates of tumor control. Outcomes in this medically inoperable group of mostly elderly patients with comorbidities were comparable with that of patients with peripheral early-stage tumors.
Trial registration: ClinicalTrials.gov NCT00750269.
Figures
Comment in
-
Stereotactic Radiation for Ultra-Central Lung Tumors: Good Idea, or Ultra-Risky?Int J Radiat Oncol Biol Phys. 2019 Mar 15;103(4):788-791. doi: 10.1016/j.ijrobp.2018.10.008. Epub 2019 Feb 20. Int J Radiat Oncol Biol Phys. 2019. PMID: 30957741 No abstract available.
-
The Search for Optimal Stereotactic Body Radiotherapy Dose in Inoperable, Centrally Located Non-Small-Cell Lung Cancer Continues.J Clin Oncol. 2019 Oct 10;37(29):2697-2699. doi: 10.1200/JCO.19.01330. Epub 2019 Aug 29. J Clin Oncol. 2019. PMID: 31465261 No abstract available.
-
Reply to T. Sio et al.J Clin Oncol. 2019 Oct 10;37(29):2699-2700. doi: 10.1200/JCO.19.01663. Epub 2019 Aug 29. J Clin Oncol. 2019. PMID: 31465262 No abstract available.
-
Delivering safe and effective stereotactic body radiation therapy for patients with centrally located early stage non-small cell lung cancer.Chin Clin Oncol. 2020 Jun;9(3):39. doi: 10.21037/cco.2019.12.17. Epub 2020 Jan 15. Chin Clin Oncol. 2020. PMID: 32008333 No abstract available.
References
-
- Videtic GMM, Donington J, Giuliani M, et al. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: Executive summary of an ASTRO evidence-based guideline. Pract Radiat Oncol. 2017;7:295–301. - PubMed
-
- Palma D, Visser O, Lagerwaard FJ, et al. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small-cell lung cancer: A population-based time-trend analysis. J Clin Oncol. 2010;28:5153–5159. - PubMed
-
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–4839. - PubMed

