Long non-coding RNA HOTAIR promotes exosome secretion by regulating RAB35 and SNAP23 in hepatocellular carcinoma
- PMID: 30943982
- PMCID: PMC6446409
- DOI: 10.1186/s12943-019-0990-6
Long non-coding RNA HOTAIR promotes exosome secretion by regulating RAB35 and SNAP23 in hepatocellular carcinoma
Abstract
Background: Emerging evidence indicates that tumor cells release a large amount of exosomes loaded with cargos during tumorigenesis. Exosome secretion is a multi-step process regulated by certain related molecules. Long non-coding RNAs (lncRNAs) play an important role in hepatocellular carcinoma (HCC) progression. However, the role of lncRNA HOTAIR in regulating exosome secretion in HCC cells remains unclear.
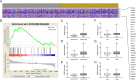
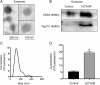
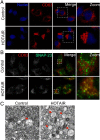
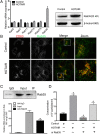
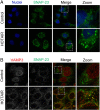
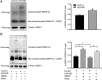
Methods: We analyzed the relationship between HOTAIR expression and exosome secretion-related genes using gene set enrichment analysis (GSEA). Nanoparticle tracking analysis was performed to validate the effect of HOTAIR on exosome secretion. The transport of multivesicular bodies (MVBs) after overexpression of HOTAIR was detected by transmission electron microscopy and confocal microscopy analysis of cluster determinant 63 (CD63) with synaptosome associated protein 23 (SNAP23). The mechanism of HOTAIR's regulation of Ras-related protein Rab-35 (RAB35), vesicle associated membrane protein 3 (VAMP3), and SNAP23 was assessed using confocal co-localization analysis, phosphorylation assays, and rescue experiments.
Results: We found an enrichment of exosome secretion-related genes in the HOTAIR high expression group. HOTAIR promoted the release of exosomes by inducing MVB transport to the plasma membrane. HOTAIR regulated RAB35 expression and localization, which controlled the docking process. Moreover, HOTAIR facilitated the final step of fusion by influencing VAMP3 and SNAP23 colocalization. In addition, we validated that HOTAIR induced the phosphorylation of SNAP23 via mammalian target of rapamycin (mTOR) signaling.
Conclusion: Our study demonstrated a novel function of lncRNA HOTAIR in promoting exosome secretion from HCC cells and provided a new understanding of lncRNAs in tumor cell biology.
Keywords: Exosome; HCC; HOTAIR; RAB35; SNAP23.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
We have obtained consents to publish this paper from all the participants of this study.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
LINC00511 drives invasive behavior in hepatocellular carcinoma by regulating exosome secretion and invadopodia formation.J Exp Clin Cancer Res. 2021 Jun 4;40(1):183. doi: 10.1186/s13046-021-01990-y. J Exp Clin Cancer Res. 2021. PMID: 34088337 Free PMC article.
-
LncRNA PVT1 promotes exosome secretion through YKT6, RAB7, and VAMP3 in pancreatic cancer.Aging (Albany NY). 2020 Jun 4;12(11):10427-10440. doi: 10.18632/aging.103268. Epub 2020 Jun 4. Aging (Albany NY). 2020. PMID: 32499447 Free PMC article.
-
Long non-coding RNA highly up-regulated in liver cancer promotes exosome secretion.World J Gastroenterol. 2019 Sep 21;25(35):5283-5299. doi: 10.3748/wjg.v25.i35.5283. World J Gastroenterol. 2019. PMID: 31558873 Free PMC article.
-
Exosomes and Hepatocellular Carcinoma: From Bench to Bedside.Int J Mol Sci. 2019 Mar 20;20(6):1406. doi: 10.3390/ijms20061406. Int J Mol Sci. 2019. PMID: 30897788 Free PMC article. Review.
-
Long noncoding RNAs: Novel insights into hepatocelluar carcinoma.Cancer Lett. 2014 Mar 1;344(1):20-27. doi: 10.1016/j.canlet.2013.10.021. Epub 2013 Oct 30. Cancer Lett. 2014. PMID: 24183851 Review.
Cited by
-
Biological functions and clinical applications of exosomal long non-coding RNAs in cancer.J Cell Mol Med. 2020 Oct;24(20):11656-11666. doi: 10.1111/jcmm.15873. Epub 2020 Sep 14. J Cell Mol Med. 2020. PMID: 32924276 Free PMC article. Review.
-
HCC-Related lncRNAs: Roles and Mechanisms.Int J Mol Sci. 2024 Jan 2;25(1):597. doi: 10.3390/ijms25010597. Int J Mol Sci. 2024. PMID: 38203767 Free PMC article. Review.
-
Advancing in Schaaf-Yang syndrome pathophysiology: from bedside to subcellular analyses of truncated MAGEL2.J Med Genet. 2023 Apr;60(4):406-415. doi: 10.1136/jmg-2022-108690. Epub 2022 Sep 7. J Med Genet. 2023. PMID: 36243518 Free PMC article.
-
A prognostic exosome-related long non-coding RNAs risk model related to the immune microenvironment and therapeutic responses for patients with liver hepatocellular carcinoma.Heliyon. 2024 Jan 10;10(2):e24462. doi: 10.1016/j.heliyon.2024.e24462. eCollection 2024 Jan 30. Heliyon. 2024. PMID: 38293480 Free PMC article.
-
Regulatory roles of noncoding RNAs in intervertebral disc degeneration as potential therapeutic targets (Review).Exp Ther Med. 2022 Dec 1;25(1):44. doi: 10.3892/etm.2022.11743. eCollection 2023 Jan. Exp Ther Med. 2022. PMID: 36569433 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

