Cytokine clearance with CytoSorb® during cardiac surgery: a pilot randomized controlled trial
- PMID: 30944029
- PMCID: PMC6448322
- DOI: 10.1186/s13054-019-2399-4
Cytokine clearance with CytoSorb® during cardiac surgery: a pilot randomized controlled trial
Abstract
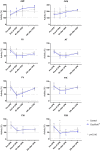
Background: Cardiopulmonary bypass (CPB) is often associated with degrees of complex inflammatory response mediated by various cytokines. This response can, in severe cases, lead to systemic hypotension and organ dysfunction. Cytokine removal might therefore improve outcomes of patients undergoing cardiac surgery. CytoSorb® (Cytosorbents, NJ, USA) is a recent device designed to remove cytokine from the blood using haemoadsorption (HA). This trial aims to evaluate the potential of CytoSorb® to decrease peri-operative cytokine levels in cardiac surgery.
Methods: We have conducted a single-centre pilot randomized controlled trial in 30 patients undergoing elective cardiac surgery and deemed at risk of complications. Patients were randomly allocated to either standard of care (n = 15) or CytoSorb® HA (n = 15) during cardiopulmonary bypass (CPB). Our primary outcome was the difference between the two groups in cytokines levels (IL-1a, IL-1b, IL-2, IL-4, IL-5, IL-6, IL-10, TNF-α, IFN-γ, MCP-1) measured at anaesthesia induction, at the end of CPB, as well as 6 and 24 h post-CPB initiation. In a consecutive subgroup of patients (10 in HA group, 11 in control group), we performed cross-adsorber as well as serial measurements of coagulation factors' activity (antithrombin, von Willebrand factor, factor II, V, VIII, IX, XI, and XII).
Results: Both groups were similar in terms of baseline and peri-operative characteristics. CytoSorb® HA during CPB was not associated with an increased incidence of adverse event. The procedure did not result in significant coagulation factors' adsorption but only some signs of coagulation activation. However, the intervention was associated neither with a decrease in pro- or anti-inflammatory cytokine levels nor with any improvement in relevant clinical outcomes.
Conclusions: CytoSorb® HA during CPB was not associated with a decrease in pro- or anti-inflammatory cytokines nor with an improvement in relevant clinical outcomes. The procedure was feasible and safe. Further studies should evaluate the efficacy of CytoSorb® HA in other clinical contexts.
Trial registration: ClinicalTrials.gov NCT02775123 . Registered 17 May 2016.
Keywords: Cardio-pulmonary bypass; Coagulation factors; CytoSorb®; Cytokines; Haemoadsorption.
Conflict of interest statement
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee Vaud (2015-00010) and registered at
Consent for publication
All patients enrolled in this trial consented for anonymous utilisation of the data in the form of a scientific publication.
Competing interests
AS has received a grant from the Leenaards foundation. He has received speaker honoraria from Cytosorbent SA. All other authors stated that they have no conflict of interest to declare.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


Comment in
-
Hemoadsorption efficacy for uncomplicated high-risk cardiac surgery.Crit Care. 2019 Nov 4;23(1):343. doi: 10.1186/s13054-019-2629-9. Crit Care. 2019. PMID: 31684994 Free PMC article. No abstract available.
References
-
- Zhang WR, Garg AX, Coca SG, Devereaux PJ, Eikelboom J, Kavsak P, McArthur E, Thiessen-Philbrook H, Shortt C, Shlipak M, et al. Plasma IL-6 and IL-10 concentrations predict AKI and long-term mortality in adults after cardiac surgery. J Am Soc Nephrol. 2015;26(12):3123–3132. doi: 10.1681/ASN.2014080764. - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

