Effect of recombinant human nerve growth factor eye drops in patients with dry eye: a phase IIa, open label, multiple-dose study
- PMID: 30944103
- PMCID: PMC6922013
- DOI: 10.1136/bjophthalmol-2018-312470
Effect of recombinant human nerve growth factor eye drops in patients with dry eye: a phase IIa, open label, multiple-dose study
Abstract
Background: Dry eye disease (DED) affects more than 14% of the elderly population causing decrease of quality of life, high costs and vision impairment. Current treatments for DED aim at lubricating and controlling inflammation of the ocular surface. Development of novel therapies targeting different pathogenic mechanisms is sought-after. The aim of this study is to evaluate safety and efficacy of recombinant human nerve growth factor (rhNGF) eye drops in patients with DED.
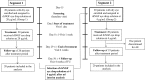
Methods: Forty consecutive patients with moderate to severe DED were included in a phase IIa, prospective, open label, multiple-dose, clinical trial to receive rhNGF eye drops at 20 µg/mL (Group 1: G1) or at 4 µg/mL (Group 2: G2) concentrations, two times a day in both eyes for 28 days (NCT02101281). The primary outcomes measures were treatment-emerged adverse events (AE), Symptoms Assessment in Dry Eye (SANDE) scale, ocular surface staining and Schirmer test.
Results: Of 40 included patients, 39 completed the trial. Both tested rhNGF eye drop concentrations were safe and well tolerated. Twenty-nine patients experienced at least one AE (14 in G1 and 15 in G2), of which 11 had at least 1 related AE (8 in G1 and 3 in G2). Both frequency and severity of DED symptoms and ocular surface damage showed significant improvement in both groups, while tear function improved only in G1.
Conclusions: The data of this study indicate that rhNGF eye drops in both doses is safe and effective in improving symptoms and signs of DED. Randomised clinical trials are ongoing to confirm the therapeutic benefit of rhNGF in DED.
Trial registration number: NCT02101281.
Keywords: clinical trial; ocular surface; pharmacology; tears.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: MS and AL: Consultant—Dompé Farmaceutici SpA. MA, FM and MF: Employee—Dompé Farmaceutici SpA. MS, AL: report personal fees from Dompé Farmaceutici SpA, Italy, outside the submitted work. MA, MS, MF, GG: report personal fees from Dompe Farmaceutici spa, during the conduct of the study.
Figures



References
-
- Bonini S, Aloe L, Bonini S, et al. . Nerve growth factor (NGF): an important molecule for trophism and healing of the ocular surface. Adv Exp Med Biol 2002;506:531–7. - PubMed
-
- Lambiase A, Manni L, Bonini S, et al. . Nerve growth factor promotes corneal healing: structural, biochemical, and molecular analyses of rat and human corneas. Invest Ophthalmol Vis Sci 2000;41:1063–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
