Assessment of Crohn's disease-associated small bowel strictures and fibrosis on cross-sectional imaging: a systematic review
- PMID: 30944110
- PMCID: PMC6580870
- DOI: 10.1136/gutjnl-2018-318081
Assessment of Crohn's disease-associated small bowel strictures and fibrosis on cross-sectional imaging: a systematic review
Abstract
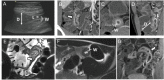
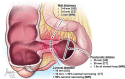
Patients with Crohn's disease commonly develop ileal and less commonly colonic strictures, containing various degrees of inflammation and fibrosis. While predominantly inflammatory strictures may benefit from a medical anti-inflammatory treatment, predominantly fibrotic strictures currently require endoscopic balloon dilation or surgery. Therefore, differentiation of the main components of a stricturing lesion is key for defining the therapeutic management. The role of endoscopy to diagnose the nature of strictures is limited by the superficial inspection of the intestinal mucosa, the lack of depth of mucosal biopsies and by the risk of sampling error due to a heterogeneous distribution of inflammation and fibrosis within a stricturing lesion. These limitations may be in part overcome by cross-sectional imaging techniques such as ultrasound, CT and MRI, allowing for a full thickness evaluation of the bowel wall and associated abnormalities. This systematic literature review provides a comprehensive summary of currently used radiologic definitions of strictures. It discusses, by assessing only manuscripts with histopathology as a gold standard, the accuracy for diagnosis of the respective modalities as well as their capability to characterise strictures in terms of inflammation and fibrosis. Definitions for strictures on cross-sectional imaging are heterogeneous; however, accuracy for stricture diagnosis is very high. Although conventional cross-sectional imaging techniques have been reported to distinguish inflammation from fibrosis and grade their severity, they are not sufficiently accurate for use in routine clinical practice. Finally, we present recent consensus recommendations and highlight experimental techniques that may overcome the limitations of current technologies.
Keywords: Crohn’s disease; fibrosis.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: DB is on the advisory board or consultant for AbbVie, Dr Falk Foundation, Ferring, MSD, Pharmacosmos, Roche, Takeda, Tillotts Pharma and Vifor. MB receives support from Siemens Healthineers in the form of salary support, hardware and software for investigating the effect of lower exposure CT in detecting active Crohn’s disease. CEP is an employee of Robarts Clinical Trials. JP has received consultancy fees from AbbVie, Arena, Boehringer Ingelheim, Galapagos, Genentech, Janssen, MSD, Novartis, Pfizer, Robarts, Second Genome, Takeda, Theravance, TiGenix and Topivert. JR is on the advisory board or consultant for Robarts Clinical Trials, Takeda and TiGenix and received research grant from AbbVie and Genentech. JGF receives grants to his institution from Siemens Healthineers and Medtronic. VJ receives salary support from the John and Susan McDonald Endowed IBD Chair at Western University, London, Ontario, Canada; consulting fees from AbbVie, Eli Lilly, GlaxoSmithKline, Arena Pharmaceuticals, Genentech, Pendopharm, Sandoz, Merck, Takeda, Janssen, Robarts Clinical Trials, Topivert and Celltrion; speaker fees from Takeda, Janssen, Shire, Ferring, AbbVie and Pfizer. BGF has received grant/research support from Millennium Pharmaceuticals, Merck, Tillotts Pharma, AbbVie, Novartis Pharmaceuticals, Centocor, Elan/Biogen, UCB Pharma, Bristol-Myers Squibb, Genentech, ActoGenix and Wyeth Pharmaceuticals; consulting fees from Millennium Pharmaceuticals, Merck, Centocor, Elan/Biogen, Janssen-Ortho, Teva Pharmaceuticals, Bristol-Myers Squibb, Celgene, UCB Pharma, AbbVie, AstraZeneca, Serono, Genentech, Tillotts Pharma, Unity Pharmaceuticals, Albireo Pharma, Given Imaging, Salix Pharmaceuticals, Novonordisk, GSK, ActoGenix, Prometheus Therapeutics and Diagnostics, Athersys, Axcan, Gilead, Pfizer, Shire, Wyeth, Zealand Pharma, Zyngenia, GiCare Pharma and Sigmoid Pharma; and speaker’s bureau fees from UCB, AbbVie and J&J/Janssen. FR is on the advisory board or consultant for AbbVie, Allergan, Celgene, Gossamer, Receptos, Thetis, UCB, Samsung, Pliant, Boehringer Ingelheim, Metacrine, Takeda, Allergan, Helmsley, RedX and Roche. RM has no conflicts of interest to declare.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
