Slow release oral morphine versus methadone for the treatment of opioid use disorder
- PMID: 30944135
- PMCID: PMC6500187
- DOI: 10.1136/bmjopen-2018-025799
Slow release oral morphine versus methadone for the treatment of opioid use disorder
Abstract
Objective: To assess the efficacy of slow release oral morphine (SROM) as a treatment for opioid use disorder (OUD).
Design: Systematic review and meta-analysis of randomised controlled trials (RCTs).
Data sources: Three electronic databases were searched through 1 May 2018: the Cochrane Central Register of Controlled Trials, MEDLINE and EMBASE. We also searched the following electronic registers for ongoing trials: ClinicalTrials.gov, WHO International Clinical Trials Registry Platform, Current Controlled Trials and the EU Clinical Trials Register.
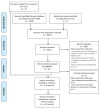
Eligibility criteria for selecting studies: We included RCTs of all durations, assessing the effect of SROM on measures of treatment retention, heroin use and craving in adults who met the diagnostic criteria for OUD.
Data extraction and synthesis: Two independent reviewers extracted data and assessed risk of bias. Data were pooled using the random-effects model and expressed as risk ratios (RRs) or mean differences with 95% CIs. Heterogeneity was assessed (χ2 statistic) and quantified (I2 statistic) and a sensitivity analysis was undertaken to assess the impact of particular high-risk trials.
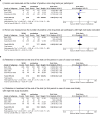
Results: Among 1315 records screened and four studies reviewed, four unique randomised trials met the inclusion criteria (n=471), and compared SROM with methadone. In the meta-analysis, we observed no significant differences between SROM and methadone in improving treatment retention (RR=0.98; 95%CI: 0.94 to 1.02, p=0.34) and heroin use (RR=0.96; 95% CI: 0.61 to 1.52, p=0.86). Craving data was not amenable to meta-analysis. Available data implied no differences in adverse events, heroin, cocaine or benzodiazepine use.
Conclusions: Meta-analysis of existing randomised trials suggests SROM may be generally equal to methadone in retaining patients in treatment and reducing heroin use while potentially resulting in less craving. The methodological quality of the included RCTs was low-to-moderate.
Keywords: meta-analysis; opioid use disorder; oral morphine; substance misuse; substance use treatment.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- NIDA. WMOverdose Death Rates: National Institute on Drug Abuse, 2017.
-
- (WHO); WHO. Guidelines for the psychosocially assisted pharmacological treatment of opioid dependence. Geneva: Switzerland, 2009. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
