Mucus accumulation in the lungs precedes structural changes and infection in children with cystic fibrosis
- PMID: 30944166
- PMCID: PMC6566903
- DOI: 10.1126/scitranslmed.aav3488
Mucus accumulation in the lungs precedes structural changes and infection in children with cystic fibrosis
Abstract
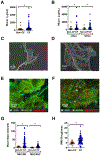
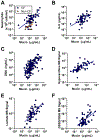
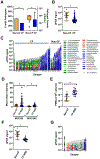
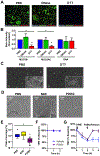
Although destructive airway disease is evident in young children with cystic fibrosis (CF), little is known about the nature of the early CF lung environment triggering the disease. To elucidate early CF pulmonary pathophysiology, we performed mucus, inflammation, metabolomic, and microbiome analyses on bronchoalveolar lavage fluid (BALF) from 46 preschool children with CF enrolled in the Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST CF) program and 16 non-CF disease controls. Total airway mucins were elevated in CF compared to non-CF BALF irrespective of infection, and higher densities of mucus flakes containing mucin 5B and mucin 5AC were observed in samples from CF patients. Total mucins and mucus flakes correlated with inflammation, hypoxia, and oxidative stress. Many CF BALFs appeared sterile by culture and molecular analyses, whereas other samples exhibiting bacterial taxa associated with the oral cavity. Children without computed tomography-defined structural lung disease exhibited elevated BALF mucus flakes and neutrophils, but little/no bacterial infection. Although CF mucus flakes appeared "permanent" because they did not dissolve in dilute BALF matrix, they could be solubilized by a previously unidentified reducing agent (P2062), but not N-acetylcysteine or deoxyribonuclease. These findings indicate that early CF lung disease is characterized by an increased mucus burden and inflammatory markers without infection or structural lung disease and suggest that mucolytic and anti-inflammatory agents should be explored as preventive therapy.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures
References
-
- Davis PB, Cystic Fibrosis Since 1938. Am. J. Respir. Crit. Care Med 173, 475–482 (2006). - PubMed
-
- Mott LS et al. , Progression of early structural lung disease in young children with cystic fibrosis assessed using CT. Thorax 67, 509–516 (2012). - PubMed
-
- Sly PD et al. , Lung disease at diagnosis in infants with cystic fibrosis detected by newborn screening. Am J Respir Crit Care Med 180, 146–152 (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

