Flavonoids enhance rod opsin stability, folding, and self-association by directly binding to ligand-free opsin and modulating its conformation
- PMID: 30944172
- PMCID: PMC6527159
- DOI: 10.1074/jbc.RA119.007808
Flavonoids enhance rod opsin stability, folding, and self-association by directly binding to ligand-free opsin and modulating its conformation
Abstract
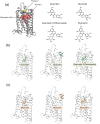
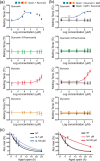
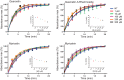
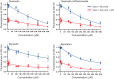
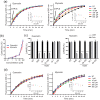
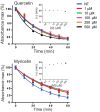
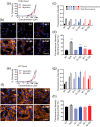
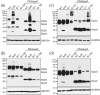
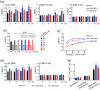
Rhodopsin (Rho) is a visual G protein-coupled receptor expressed in the rod photoreceptors of the eye, where it mediates transmission of a light signal into a cell and converts this signal into a nerve impulse. More than 100 mutations in Rho are linked to various ocular impairments, including retinitis pigmentosa (RP). Accordingly, much effort has been directed toward developing ligands that target Rho and improve its folding and stability. Natural compounds may provide another viable approach to such drug discovery efforts. The dietary polyphenol compounds, ubiquitously present in fruits and vegetables, have beneficial effects in several eye diseases. However, the underlying mechanism of their activity is not fully understood. In this study, we used a combination of computational methods, biochemical and biophysical approaches, including bioluminescence resonance energy transfer, and mammalian cell expression systems to clarify the effects of four common bioactive flavonoids (quercetin, myricetin, and their mono-glycosylated forms quercetin-3-rhamnoside and myricetrin) on rod opsin stability, function, and membrane organization. We observed that by directly interacting with ligand-free opsin, flavonoids modulate its conformation, thereby causing faster entry of the retinal chromophore into its binding pocket. Moreover, flavonoids significantly increased opsin stability, most likely by introducing structural rigidity and promoting receptor self-association within the biological membranes. Of note, the binding of flavonoids to an RP-linked P23H opsin variant partially restored its normal cellular trafficking. Together, our results suggest that flavonoids could be utilized as lead compounds in the development of effective nonretinoid therapeutics for managing RP-related retinopathies.
Keywords: G protein-coupled receptor (GPCR); eye disease; flavonoid; oligomerization; opsin; photoreception; receptor; regeneration; rhodopsin; stability.
© 2019 Ortega et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

