Envelope Protein Glycosylation Mediates Zika Virus Pathogenesis
- PMID: 30944176
- PMCID: PMC6613755
- DOI: 10.1128/JVI.00113-19
Envelope Protein Glycosylation Mediates Zika Virus Pathogenesis
Abstract
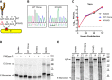
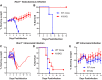
Zika virus (ZIKV) is an emerging mosquito-borne flavivirus. Recent ZIKV outbreaks have produced serious human disease, including neurodevelopmental malformations (congenital Zika syndrome) and Guillain-Barré syndrome. These outcomes were not associated with ZIKV infection prior to 2013, raising the possibility that viral genetic changes could contribute to new clinical manifestations. All contemporary ZIKV isolates encode an N-linked glycosylation site in the envelope (E) protein (N154), but this glycosylation site is absent in many historical ZIKV isolates. Here, we investigated the role of E protein glycosylation in ZIKV pathogenesis using two contemporary Asian-lineage strains (H/PF/2013 and PRVABC59) and the historical African-lineage strain (MR766). We found that glycosylated viruses were highly pathogenic in Ifnar1-/- mice. In contrast, nonglycosylated viruses were attenuated, producing lower viral loads in the serum and brain when inoculated subcutaneously but remaining neurovirulent when inoculated intracranially. These results suggest that E glycosylation is advantageous in the periphery but not within the brain. Accordingly, we found that glycosylation facilitated infection of cells expressing the lectins dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN) or DC-SIGN-related (DC-SIGNR), suggesting that inefficient infection of lectin-expressing leukocytes could contribute to the attenuation of nonglycosylated ZIKV in mice.IMPORTANCE It is unclear why the ability of Zika virus (ZIKV) to cause serious disease, including Guillain-Barré syndrome and birth defects, was not recognized until recent outbreaks. One contributing factor could be genetic differences between contemporary ZIKV strains and historical ZIKV strains. All isolates from recent outbreaks encode a viral envelope protein that is glycosylated, whereas many historical ZIKV strains lack this glycosylation. We generated nonglycosylated ZIKV mutants from contemporary and historical strains and evaluated their virulence in mice. We found that nonglycosylated viruses were attenuated and produced lower viral loads in serum and brains. Our studies suggest that envelope protein glycosylation contributes to ZIKV pathogenesis, possibly by facilitating attachment to and infection of lectin-expressing leukocytes.
Keywords: CD209; CD209L; DC-SIGN; DC-SIGNR; Ifnar1−/− mouse; L-SIGN; Zika virus; flavivirus; glycosylation.
Copyright © 2019 American Society for Microbiology.
Figures



Similar articles
-
Zika Virus Encoding Nonglycosylated Envelope Protein Is Attenuated and Defective in Neuroinvasion.J Virol. 2017 Nov 14;91(23):e01348-17. doi: 10.1128/JVI.01348-17. Print 2017 Dec 1. J Virol. 2017. PMID: 28931684 Free PMC article.
-
The Envelope Residues E152/156/158 of Zika Virus Influence the Early Stages of Virus Infection in Human Cells.Cells. 2019 Nov 15;8(11):1444. doi: 10.3390/cells8111444. Cells. 2019. PMID: 31731738 Free PMC article.
-
Two Genetic Differences between Closely Related Zika Virus Strains Determine Pathogenic Outcome in Mice.J Virol. 2020 Sep 29;94(20):e00618-20. doi: 10.1128/JVI.00618-20. Print 2020 Sep 29. J Virol. 2020. PMID: 32796074 Free PMC article.
-
Structures of Zika Virus E & NS1: Relations with Virus Infection and Host Immune Responses.Adv Exp Med Biol. 2018;1062:77-87. doi: 10.1007/978-981-10-8727-1_6. Adv Exp Med Biol. 2018. PMID: 29845526 Review.
-
Flavivirus Envelope Protein Glycosylation: Impacts on Viral Infection and Pathogenesis.J Virol. 2020 May 18;94(11):e00104-20. doi: 10.1128/JVI.00104-20. Print 2020 May 18. J Virol. 2020. PMID: 32161171 Free PMC article. Review.
Cited by
-
Insights into Zika Virus Pathogenesis and Potential Therapeutic Strategies.Biomedicines. 2023 Dec 15;11(12):3316. doi: 10.3390/biomedicines11123316. Biomedicines. 2023. PMID: 38137537 Free PMC article. Review.
-
A Zika virus-specific IgM elicited in pregnancy exhibits ultrapotent neutralization.Cell. 2022 Dec 8;185(25):4826-4840.e17. doi: 10.1016/j.cell.2022.10.023. Epub 2022 Nov 18. Cell. 2022. PMID: 36402135 Free PMC article.
-
DC/L-SIGN recognition of spike glycoprotein promotes SARS-CoV-2 trans-infection and can be inhibited by a glycomimetic antagonist.PLoS Pathog. 2021 May 20;17(5):e1009576. doi: 10.1371/journal.ppat.1009576. eCollection 2021 May. PLoS Pathog. 2021. PMID: 34015061 Free PMC article.
-
Transmission of Zika virus by dendritic cell subsets in skin and vaginal mucosa.Front Immunol. 2023 Mar 6;14:1125565. doi: 10.3389/fimmu.2023.1125565. eCollection 2023. Front Immunol. 2023. PMID: 36949942 Free PMC article.
-
Establishment of a CPER reverse genetics system for Powassan virus defines attenuating NS1 glycosylation sites and an infectious NS1-GFP11 reporter virus.mBio. 2023 Aug 31;14(4):e0138823. doi: 10.1128/mbio.01388-23. Epub 2023 Jul 25. mBio. 2023. PMID: 37489888 Free PMC article.
References
-
- Oehler E, Watrin L, Larre P, Leparc-Goffart I, Lastere S, Valour F, Baudouin L, Mallet H, Musso D, Ghawche F. 2014. Zika virus infection complicated by Guillain-Barre syndrome—case report, French Polynesia, December 2013. Euro Surveill 19:20720. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

