Cone photoreceptor classification in the living human eye from photostimulation-induced phase dynamics
- PMID: 30944223
- PMCID: PMC6475411
- DOI: 10.1073/pnas.1816360116
Cone photoreceptor classification in the living human eye from photostimulation-induced phase dynamics
Abstract
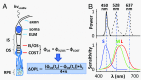
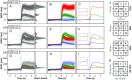
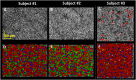
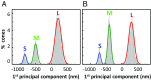
Human color vision is achieved by mixing neural signals from cone photoreceptors sensitive to different wavelengths of light. The spatial arrangement and proportion of these spectral types in the retina set fundamental limits on color perception, and abnormal or missing types are responsible for color vision loss. Imaging provides the most direct and quantitative means to study these photoreceptor properties at the cellular scale in the living human retina, but remains challenging. Current methods rely on retinal densitometry to distinguish cone types, a prohibitively slow process. Here, we show that photostimulation-induced optical phase changes occur in cone cells and carry substantial information about spectral type, enabling cones to be differentiated with unprecedented accuracy and efficiency. Moreover, these phase dynamics arise from physiological activity occurring on dramatically different timescales (from milliseconds to seconds) inside the cone outer segment, thus exposing the phototransduction cascade and subsequent downstream effects. We captured these dynamics in cones of subjects with normal color vision and a deuteranope, and at different macular locations by: (i) marrying adaptive optics to phase-sensitive optical coherence tomography to avoid optical blurring of the eye, (ii) acquiring images at high speed that samples phase dynamics at up to 3 KHz, and (iii) localizing phase changes to the cone outer segment, where photoactivation occurs. Our method should have broad appeal for color vision applications in which the underlying neural processing of photoreceptors is sought and for investigations of retinal diseases that affect cone function.
Keywords: adaptive optics; color vision; cone classification; optical coherence tomography; retina.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Rodieck RW. The First Steps in Seeing. Sinauer Associates; Sunderland, MA: 1998.
-
- Greenstein VC, Hood DC, Ritch R, Steinberger D, Carr RE. S (blue) cone pathway vulnerability in retinitis pigmentosa, diabetes and glaucoma. Invest Ophthalmol Vis Sci. 1989;30:1732–1737. - PubMed
-
- Sharpe LT, Stockman A, Jägle H, Nathans J. Opsin genes, cone photopigments, color vision, and color blindness. In: Gegenfurtner KR, Sharp TL, editors. Color Vision: From Genes to Perception. Cambridge Univ Press; Cambridge, UK: 1999. pp. 3–51.
-
- De Vries H. The heredity of the relative numbers of red and green receptors in the human eye. Genetica. 1949;24:199–212.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

