Altered X-chromosome inactivation in T cells may promote sex-biased autoimmune diseases
- PMID: 30944248
- PMCID: PMC6483655
- DOI: 10.1172/jci.insight.126751
Altered X-chromosome inactivation in T cells may promote sex-biased autoimmune diseases
Abstract
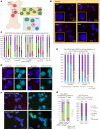
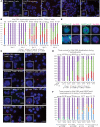
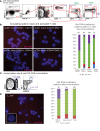
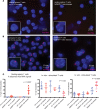
Systemic lupus erythematosus (SLE) is an autoimmune disorder that predominantly affects women and is driven by autoreactive T cell-mediated inflammation. It is known that individuals with multiple X-chromosomes are at increased risk for developing SLE; however, the mechanisms underlying this genetic basis are unclear. Here, we use single cell imaging to determine the epigenetic features of the inactive X (Xi) in developing thymocytes, mature T cell subsets, and T cells from SLE patients and mice. We show that Xist RNA and heterochromatin modifications transiently reappear at the Xi and are missing in mature single positive T cells. Activation of mature T cells restores Xist RNA and heterochromatin marks simultaneously back to the Xi. Notably, X-chromosome inactivation (XCI) maintenance is altered in T cells of SLE patients and late-stage-disease NZB/W F1 female mice, and we show that X-linked genes are abnormally upregulated in SLE patient T cells. SLE T cells also have altered expression of XIST RNA interactome genes, accounting for perturbations of Xi epigenetic features. Thus, abnormal XCI maintenance is a feature of SLE disease, and we propose that Xist RNA localization at the Xi could be an important factor for maintaining dosage compensation of X-linked genes in T cells.
Keywords: Autoimmunity; Cell Biology; Epigenetics; Noncoding RNAs; T cell development.
Conflict of interest statement
Figures



Comment in
-
X-chromosome inactivation altered in SLE.Nat Rev Rheumatol. 2019 Jun;15(6):318. doi: 10.1038/s41584-019-0226-6. Nat Rev Rheumatol. 2019. PMID: 31040440 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

