Vimentin intermediate filament assembly regulates fibroblast invasion in fibrogenic lung injury
- PMID: 30944258
- PMCID: PMC6483650
- DOI: 10.1172/jci.insight.123253
Vimentin intermediate filament assembly regulates fibroblast invasion in fibrogenic lung injury
Abstract
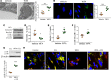
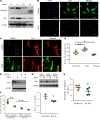
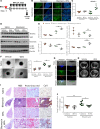
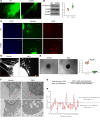
Idiopathic pulmonary fibrosis (IPF) is a progressive disease, with a median survival of 3-5 years following diagnosis. Lung remodeling by invasive fibroblasts is a hallmark of IPF. In this study, we demonstrate that inhibition of vimentin intermediate filaments (VimIFs) decreases the invasiveness of IPF fibroblasts and confers protection against fibrosis in a murine model of experimental lung injury. Increased expression and organization of VimIFs contribute to the invasive property of IPF fibroblasts in connection with deficient cellular autophagy. Blocking VimIF assembly by pharmacologic and genetic means also increases autophagic clearance of collagen type I. Furthermore, inhibition of expression of collagen type I by siRNA decreased invasiveness of fibroblasts. In a bleomycin injury model, enhancing autophagy in fibroblasts by an inhibitor of VimIF assembly, withaferin A (WFA), protected from fibrotic lung injury. Additionally, in 3D lung organoids, or pulmospheres, from patients with IPF, WFA reduced the invasiveness of lung fibroblasts in the majority of subjects tested. These studies provide insights into the functional role of vimentin, which regulates autophagy and restricts the invasiveness of lung fibroblasts.
Keywords: Autophagy; Drug therapy; Fibrosis; Pulmonology.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

