Gas phase synthesis of [4]-helicene
- PMID: 30944302
- PMCID: PMC6447558
- DOI: 10.1038/s41467-019-09224-8
Gas phase synthesis of [4]-helicene
Abstract
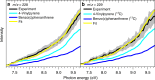
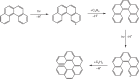
A synthetic route to racemic helicenes via a vinylacetylene mediated gas phase chemistry involving elementary reactions with aryl radicals is presented. In contrast to traditional synthetic routes involving solution chemistry and ionic reaction intermediates, the gas phase synthesis involves a targeted ring annulation involving free radical intermediates. Exploiting the simplest helicene as a benchmark, we show that the gas phase reaction of the 4-phenanthrenyl radical ([C14H9]•) with vinylacetylene (C4H4) yields [4]-helicene (C18H12) along with atomic hydrogen via a low-barrier mechanism through a resonance-stabilized free radical intermediate (C18H13). This pathway may represent a versatile mechanism to build up even more complex polycyclic aromatic hydrocarbons such as [5]- and [6]-helicene via stepwise ring annulation through bimolecular gas phase reactions in circumstellar envelopes of carbon-rich stars, whereas secondary reactions involving hydrogen atom assisted isomerization of thermodynamically less stable isomers of [4]-helicene might be important in combustion flames as well.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Chen, C.-F. & Shen, Y. Helicene Chemistry: From Synthesis to Applications Ch. 1 (Springer, 2016).
-
- Bruyère A, et al. Intermixing of chirality and local structure in the second harmonic generation response of dibenzo[c]acridine helicene-like molecule thin films. J. Phys. Chem. C. 2017;121:24759–24765. doi: 10.1021/acs.jpcc.7b07401. - DOI
-
- Hoffmann N. Photochemical reactions applied to the synthesis of helicenes and helicene-like compounds. J. Photochem. Photobiol. C. 2014;19:1–19. doi: 10.1016/j.jphotochemrev.2013.11.001. - DOI

