Impact of drought stress on simultaneously occurring pathogen infection in field-grown chickpea
- PMID: 30944350
- PMCID: PMC6447570
- DOI: 10.1038/s41598-019-41463-z
Impact of drought stress on simultaneously occurring pathogen infection in field-grown chickpea
Abstract
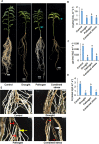
Drought stress and pathogen infection simultaneously occur in the field. In this study, the interaction of these two stresses with chickpea, their individual and combined effect and the net impact on plant growth and yield traits were systematically assessed under field and confined pot experiments. The field experiments were conducted for four consecutive years from 2014-15 to 2017-18 at different locations of India. Different irrigation regimes were maintained to impose mild to severe drought stress, and natural incidence of the pathogen was considered as pathogen stress. We observed an increased incidence of fungal diseases namely, dry root rot (DRR) caused by Rhizoctonia bataticola, black root rot (BRR) caused by Fusarium solani under severe drought stress compared to well-irrigated field condition. Similar to field experiments, pot experiments also showed severe disease symptoms of DRR and BRR in the presence of drought compared to pathogen only stress. Overall, the results from this study not only showed the impact of combined drought and DRR stress but also provided systematic data, first of its kind, for the use of researchers.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Kumar, J. & Abbo, S. Genetics of flowering time in chickpea and its bearing on productivity in semiarid environments in Advances in Agronomy (ed. Spaks, D. L.) 122–124. ISBN 978-0-12-374361-9 (Academic Press 2001).
-
- Ahmad, F., Gaur, P. & Croser, J. Chickpea (Cicer arietinum L.) in Genetic Resources, Chromosome Engineering, and Crop Improvement (ed. Ram J. Singh) 229–267. ISBN 9780849336393 (CRC Press: Boca Raton, F. L., Taylor & Francis, London, UK. 2006).
-
- Gaur, P. M. et al. Climate change and heat stress tolerance in chickpea in Climate Change and Plant Abiotic Stress Tolerance (eds N. Tuteja and S. S. Gill) 837–856; 10.1002/9783527675265.ch31 (Wiley‐VCH Verlag GmbH & Co. 2013).
-
- Devasirvatham V, Tan D. Impact of high temperature and drought stresses on chickpea production. Agronomy. 2018;8:145. doi: 10.3390/agronomy8080145. - DOI
-
- Nene, Y. et al. Field diagnosis of chickpea diseases and their control. Information Bulletin No. 28 (revised). (Technical Report, International Crops Research Institute for the Semi-Arid Tropics), http://oar.icrisat.org/6601/1/InfoBulletin_28-ICRISAT_2012.pdf (2012).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

