Pulsed immune reconstitution therapy in multiple sclerosis
- PMID: 30944586
- PMCID: PMC6440030
- DOI: 10.1177/1756286419836913
Pulsed immune reconstitution therapy in multiple sclerosis
Abstract
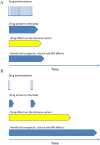
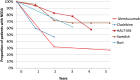
Whereas drugs used for maintenance/escalation therapy do not maintain their beneficial effect after cessation of therapy, some new highly effective therapies can show prolonged treatment effects after a short treatment course. Such therapies have been named pulsed immune reconstitution therapies or pulsed immunosuppressive therapies, and typical representatives are alemtuzumab and cladribine. Autologous haematopoietic stem cell transplantation could be considered as the strongest immune reconstitution therapy. Both alemtuzumab and cladribine induce depletion of lymphocytes, and a common mechanism of action is preferential depletion of class-switched and unswitched memory B-cells. Whereas CD-19+ B-lymphocytes repopulate within 6 months, CD4+ T-cells repopulate at a slower rate, taking 1-2 years to reach the lower level of normal. In general, the depletion of lymphocytes is more profound and the repletion of T-cells is slower after alemtuzumab than after cladribine treatment. Both drugs have a strong effect on relapses and magnetic resonance imaging (MRI) activity, and reduce disability worsening. The therapeutic effect is maintained beyond the period of active treatment in a large proportion of patients, which is best documented for alemtuzumab. Adverse effects include reactivation of latent infections such as tuberculosis and risk of herpes zoster. The main disadvantage in alemtuzumab-treated patients is the risk of secondary immune-mediated disorders. Pulsed immune reconstitution therapy is an option as initial therapy in relapsing-remitting multiple sclerosis patients with high disease activity and in patients on treatment with another disease-modifying therapy with significant relapse and/or MRI activity.
Keywords: MS; alemtuzumab; autologous haematopoietic stem cell transplantation; cladribine; multiple sclerosis; multiple sclerosis treatment; pulsed immune reconstitution therapy.
Conflict of interest statement
Conflict of interest statement: PSS has received personal compensation for serving on scientific advisory boards, steering committees or independent data monitoring boards for Biogen, Merck, Novartis, TEVA, GlaxoSmithKline, MedDay Pharmaceuticals, Genzyme, Celgene and Forward Pharma, and has received speaker honoraria from Biogen, Merck, Teva, Genzyme and Novartis. FS has served on scientific advisory boards, been on the steering committees of clinical trials, served as a consultant, received support for congress participation, received speaker honoraria or received research support for his laboratory from Biogen, EMD Serono, Merck, Novartis, Roche and Sanofi Genzyme.
Figures

References
-
- Sorensen PS. New management algorithms in multiple sclerosis. Curr Opin Neurol 2014; 27: 246–259. - PubMed
-
- Comi G, Radaelli M, Soelberg Sorensen P. Evolving concepts in the treatment of relapsing multiple sclerosis. Lancet 2017; 389: 1347–1356. - PubMed
-
- Sorensen PS, Koch-Henriksen N, Petersen T, et al. Recurrence or rebound of clinical relapses after discontinuation of natalizumab therapy in highly active MS patients. J Neurol 2014; 261: 1170–1177. - PubMed
-
- Wiendl H. Cladribine: an old newcomer for pulsed immune reconstitution in MS. Nat Rev Neurol 2017; 13: 573–574. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

