Non-invasive imaging techniques for the in vivo diagnosis of Bowen's disease: Three case reports
- PMID: 30944602
- PMCID: PMC6444281
- DOI: 10.3892/ol.2019.10079
Non-invasive imaging techniques for the in vivo diagnosis of Bowen's disease: Three case reports
Abstract
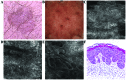
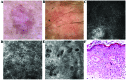
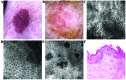
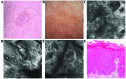
Bowen's disease (BD) is a relatively frequent non-melanoma skin cancer occurring mostly in elderly people. Until now, the usual way to establish the diagnosis is histopathological examination of a skin biopsy. Dermoscopy and reflectance confocal microscopy (RCM) are modern alternative methods that can be used as quick and non-invasive diagnostic techniques and as follow-up instruments in cases in which a conservative treatment is chosen for the management of BD. There are no very specific dermoscopic criteria for the diagnosis of this disease, but some dermoscopic features (scaly surface, vascular structures and pigmentation) can be found more frequent and can be helpful for the diagnosis. RCM of BD shows an acanthotic epidermis with two types of targetoid cells: the first, a large cell with bright center and dark peripheral halo, the second, a cell with dark center and a bright rim surrounded by a dark hallo, related with dyskeratotic cells on histological examination. BD management could be improved by using non-invasive, in vivo imaging techniques that allow a fast and easy diagnosis and can be used as follow-up tools. However, larger studies are necessary for the validation of our observations.
Keywords: Bowen's disease; dermoscopy; diagnosis; in vivo; non-invasive; reflectance confocal microscopy.
Figures
References
LinkOut - more resources
Full Text Sources
