Intermolecular dialkylation of alkenes with two distinct C(sp3)─H bonds enabled by synergistic photoredox catalysis and iron catalysis
- PMID: 30944866
- PMCID: PMC6440757
- DOI: 10.1126/sciadv.aav9839
Intermolecular dialkylation of alkenes with two distinct C(sp3)─H bonds enabled by synergistic photoredox catalysis and iron catalysis
Abstract
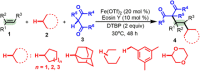
The functionalization of unactivated C(sp3)─H bonds represents one of the most powerful and most atom-economical tools for the formation of new carbon-based chemical bonds in synthesis. Although cross-dehydrogenative coupling reactions of two distinct C─H bonds for the formation of carbon-carbon bonds have been well investigated, controlled functionalizations of two or more different C(sp3)─H bonds across a functional group or a molecule (e.g., an alkene or alkyne) in a single reaction remain challenging. Here, we present a three-component dialkylation of alkenes with common alkanes and 1,3-dicarbonyl compounds via synergistic photoredox catalysis and iron catalysis for the synthesis of two functionalized 1,3-dicarbonyl compounds. Mechanistic studies suggest that the photoredox catalysis serves as a promotion system to allow the dialkylation to proceed under mild conditions by reducing the oxidation and reduction potentials of the iron intermediates and the reaction partners.
Figures
References
-
- Tang S., Liu K., Liu C., Lei A., Olefinic C–H functionalization through radical alkenylation. Chem. Soc. Rev. 44, 1070–1082 (2015). - PubMed
-
- Dhungana R. K., KC S., Basnet P., Giri R., Transition metal-catalyzed dicarbofunctionalization of unactivated olefins. Chem. Rec. 18, 1314–1340 (2018). - PubMed
-
- Basnet P., KC S., Dhungana R. K., Shrestha B., Boyle T. J., Giri R., Synergistic bimetallic Ni/Ag and Ni/Cu catalysis for regioselective γ,δ-diarylation of alkenyl ketimines: Addressing β-H elimination by in situ generation of cationic Ni(II) Catalysts. J. Am. Chem. Soc. 140, 15586–15590 (2018). - PubMed
LinkOut - more resources
Full Text Sources

