Second-line treatments in children with immune thrombocytopenia: Effect on platelet count and patient-centered outcomes
- PMID: 30945320
- PMCID: PMC6527349
- DOI: 10.1002/ajh.25479
Second-line treatments in children with immune thrombocytopenia: Effect on platelet count and patient-centered outcomes
Abstract
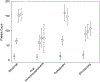
Immune thrombocytopenia (ITP) is an autoimmune bleeding disorder with isolated thrombocytopenia and hemorrhagic risk. While many children with ITP can be safely observed, treatments are often needed for various reasons, including to decrease bleeding, or to improve health related quality of life (HRQoL). There are a number of available second-line treatments, including rituximab, thrombopoietin-receptor agonists, oral immunosuppressive agents, and splenectomy, but data comparing treatment outcomes are lacking. ICON1 is a prospective, multi-center, observational study of 120 children starting second-line treatments for ITP designed to compare treatment outcomes including platelet count, bleeding, and HRQoL utilizing the Kids ITP Tool (KIT). While all treatments resulted in increased platelet counts, romiplostim had the most pronounced effect at 6 months (P = .04). Only patients on romiplostim and rituximab had a significant reduction in both skin-related (84% to 48%, P = .01 and 81% to 43%, P = .004) and non-skin-related bleeding symptoms (58% to 14%, P = .0001 and 54% to 17%, P = .0006) after 1 month of treatment. HRQoL significantly improved on all treatments. However, only patients treated with eltrombopag had a median improvement in KIT scores at 1 month that met the minimal important difference (MID). Bleeding, platelet count, and HRQoL improved in each treatment group, but the extent and timing of the effect varied among treatments. These results are hypothesis generating and help to improve our understanding of the effect of each treatment on specific patient outcomes. Combined with future randomized trials, these findings will help clinicians select the optimal second-line treatment for an individual child with ITP.
© 2019 Wiley Periodicals, Inc.
Conflict of interest statement
Figures
References
-
- Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386–2393. - PubMed
-
- Neunert C, Lim W, Crowther M, et al. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117(16):4190–4207. - PubMed
-
- Schifferli A, Holbro A, Chitlur M, et al. A comparative prospective observational study of children and adults with immune thrombocytopenia: 2-year follow-up. American journal of hematology. 2018;93(6):751–759. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous