Developmental origins and oncogenic pathways in malignant brain tumors
- PMID: 30945456
- PMCID: PMC6565468
- DOI: 10.1002/wdev.342
Developmental origins and oncogenic pathways in malignant brain tumors
Abstract
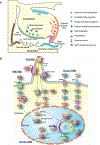
Brain tumors such as adult glioblastomas and pediatric high-grade gliomas or medulloblastomas are among the leading causes of cancer-related deaths, exhibiting poor prognoses with little improvement in outcomes in the past several decades. These tumors are heterogeneous and can be initiated from various neural cell types, contributing to therapy resistance. How such heterogeneity arises is linked to the tumor cell of origin and their genetic alterations. Brain tumorigenesis and progression recapitulate key features associated with normal neurogenesis; however, the underlying mechanisms are quite dysregulated as tumor cells grow and divide in an uncontrolled manner. Recent comprehensive genomic, transcriptomic, and epigenomic studies at single-cell resolution have shed new light onto diverse tumor-driving events, cellular heterogeneity, and cells of origin in different brain tumors. Primary and secondary glioblastomas develop through different genetic alterations and pathways, such as EGFR amplification and IDH1/2 or TP53 mutation, respectively. Mutations such as histone H3K27M impacting epigenetic modifications define a distinct group of pediatric high-grade gliomas such as diffuse intrinsic pontine glioma. The identification of distinct genetic, epigenomic profiles and cellular heterogeneity has led to new classifications of adult and pediatric brain tumor subtypes, affording insights into molecular and lineage-specific vulnerabilities for treatment stratification. This review discusses our current understanding of tumor cells of origin, heterogeneity, recurring genetic and epigenetic alterations, oncogenic drivers and signaling pathways for adult glioblastomas, pediatric high-grade gliomas, and medulloblastomas, the genetically heterogeneous groups of malignant brain tumors. This article is categorized under: Gene Expression and Transcriptional Hierarchies > Gene Networks and Genomics Adult Stem Cells, Tissue Renewal, and Regeneration > Stem Cell Differentiation and Reversion Signaling Pathways > Cell Fate Signaling.
Keywords: epigenetic regulation; glial progenitor cells; glioma; medulloblastoma; neural stem cells; oligodendrocyte progenitors; oncogenic signaling network; tumor suppressors.
© 2019 Wiley Periodicals, Inc.
Conflict of interest statement
Declaration of Interests
The authors declare no competing interests.
Figures

References
-
- Archer TC, Ehrenberger T, Mundt F, Gold MP, Krug K, Mah CK, Mahoney EL, Daniel CJ, LeNail A, Ramamoorthy D, Mertins P, Mani DR, Zhang H, Gillette MA, Clauser K, Noble M, Tang LC, Pierre-Francois J, Silterra J, Jensen J, Tamayo P, Korshunov A, Pfister SM, Kool M, Northcott PA, Sears RC, Lipton JO, Carr SA, Mesirov JP, Pomeroy SL, Fraenkel E, 2018. Proteomics, Post-translational Modifications, and Integrative Analyses Reveal Molecular Heterogeneity within Medulloblastoma Subgroups. Cancer Cell 34, 396–410 e398. - PMC - PubMed
-
- Bachoo RM, Maher EA, Ligon KL, Sharpless NE, Chan SS, You MJ, Tang Y, DeFrances J, Stover E, Weissleder R, Rowitch DH, Louis DN, DePinho RA, 2002. Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell 1, 269–277. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

