Novel functions of macrophages in the heart: insights into electrical conduction, stress, and diastolic dysfunction
- PMID: 30945736
- PMCID: PMC7778432
- DOI: 10.1093/eurheartj/ehz159
Novel functions of macrophages in the heart: insights into electrical conduction, stress, and diastolic dysfunction
Abstract
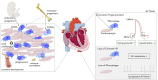
Over a century ago, Élie Metchnikoff described the macrophages' ability to phagocytose. Propelled by advances in technology enabling phenotypic and functional analyses at unpreceded resolution, a recent renaissance in macrophage research has shed new light on these 'big eaters'. We here give an overview of cardiac macrophages' provenance in the contexts of cardiac homeostasis and stress. We highlight the recently identified mechanism by which these cells regulate electrical conduction in the atrioventricular node and discuss why we need a deeper understanding of monocytes and macrophages in systolic and diastolic dysfunctions.
Keywords: Conduction; Diastolic dysfunction; Macrophages; Monocytes; Myocardial infarction.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author(s) 2019. For permissions, please email: journals.permissions@oup.com.
Figures

References
-
- Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 2000;404:193–197. - PubMed
-
- Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, Cumano A, Geissmann F. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science 2006;311:83–87. - PubMed
-
- Hettinger J, Richards DM, Hansson J, Barra MM, Joschko AC, Krijgsveld J, Feuerer M. Origin of monocytes and macrophages in a committed progenitor. Nat Immunol 2013;14:821–830. - PubMed

