Stiripentol protects against calcium oxalate nephrolithiasis and ethylene glycol poisoning
- PMID: 30946030
- PMCID: PMC6538379
- DOI: 10.1172/JCI99822
Stiripentol protects against calcium oxalate nephrolithiasis and ethylene glycol poisoning
Abstract
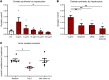
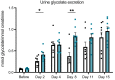
Increased urinary oxalate excretion (hyperoxaluria) promotes the formation of calcium oxalate crystals. Monogenic diseases due to hepatic enzymes deficiency result in chronic hyperoxaluria, promoting end-stage renal disease in children and young adults. Ethylene glycol poisoning also results in hyperoxaluria promoting acute renal failure and frequently death. Stiripentol is an antiepileptic drug used to treat children affected by Dravet syndrome, possibly by inhibiting neuronal lactate dehydrogenase 5 isoenzyme. As this isoenzyme is also the last step of hepatic oxalate production, we hypothesized that Stiripentol would potentially reduce hepatic oxalate production and urine oxalate excretion. In vitro, Stiripentol decreased in a dose-dependent manner the synthesis of oxalate by hepatocytes. In vivo, Stiripentol oral administration reduced significantly urine oxalate excretion in rats. Stiripentol protected kidneys against calcium oxalate crystal deposits in acute ethylene glycol intoxication and chronic calcium oxalate nephropathy models. In both models, Stiripentol improved significantly renal function. Patients affected by Dravet syndrome and treated with Stiripentol had a lower urine oxalate excretion than control patients. A young girl affected by severe type I hyperoxaluria received Stiripentol for several weeks: urine oxalate excretion decreased by two-thirds. Stiripentol is a promising potential therapy against genetic hyperoxaluria and ethylene glycol poisoning.
Keywords: Nephrology; Urology.
Conflict of interest statement
Figures



Comment in
-
Lactate dehydrogenase 5: identification of a druggable target to reduce oxaluria.J Clin Invest. 2019 May 20;129(6):2201-2204. doi: 10.1172/JCI128709. eCollection 2019 May 20. J Clin Invest. 2019. PMID: 31107247 Free PMC article.
-
Re: Stiripentol Protects against Calcium Oxalate Nephrolithiasis and Ethylene Glycol Poisoning.J Urol. 2019 Nov;202(5):862. doi: 10.1097/01.JU.0000579360.86322.4f. Epub 2019 Oct 9. J Urol. 2019. PMID: 31364941 No abstract available.
-
Stiripentol for the treatment of primary hyperoxaluria and calcium oxalate nephropathy.Kidney Int. 2020 Jan;97(1):17-19. doi: 10.1016/j.kint.2019.06.011. Epub 2019 Aug 23. Kidney Int. 2020. PMID: 31451300 No abstract available.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

