The landscape of the mesenchymal signature in brain tumours
- PMID: 30946477
- PMCID: PMC6485274
- DOI: 10.1093/brain/awz044
The landscape of the mesenchymal signature in brain tumours
Abstract
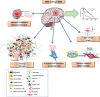
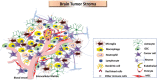
The complexity of glioblastoma multiforme, the most common and lethal variant of gliomas, is reflected by cellular and molecular heterogeneity at both the inter- and intra-tumoural levels. Molecular subtyping has arisen in the past two decades as a promising strategy to give better predictions of glioblastoma multiforme evolution, common disease pathways, and rational treatment options. The Cancer Genome Atlas network initially identified four molecular subtypes of glioblastoma multiforme: proneural, neural, mesenchymal and classical. However, further studies, also investigated glioma stem cells, have only identified two to three subtypes: proneural, mesenchymal and classical. The proneural-mesenchymal transition upon tumour recurrence has been suggested as a mechanism of tumour resistance to radiation and chemotherapy treatment. Glioblastoma multiforme patients with the mesenchymal subtype tend to survive shorter than other subtypes when analysis is restricted to samples with low transcriptional heterogeneity. Although the mesenchymal signature in malignant glioma may seem at odds with the common idea of the ectodermal origin of neural-glial lineages, the presence of the mesenchymal signature in glioma is supported by several studies suggesting that it can result from: (i) intrinsic expression of tumour cells affected with accumulated genetic mutations and cell of origin; (ii) tumour micro-environments with recruited macrophages or microglia, mesenchymal stem cells or pericytes, and other progenitors; (iii) resistance to tumour treatment, including radiotherapy, antiangiogenic therapy and possibly chemotherapy. Genetic abnormalities, mainly NF1 mutations, together with NF-κB transcriptional programs, are the main driver of acquiring mesenchymal-signature. This signature is far from being simply tissue artefacts, as it has been identified in single cell glioma, circulating tumour cells, and glioma stem cells that are released from the tumour micro-environment. All these together suggest that the mesenchymal signature in glioblastoma multiforme is induced and sustained via cell intrinsic mechanisms and tumour micro-environment factors. Although patients with the mesenchymal subtype tend to have poorer prognosis, they may have favourable response to immunotherapy and intensive radio- and chemotherapy.
Keywords: glioma; mesenchymal subtype; proneural-mesenchymal transition; subtype origin; tumor microenvironment.
© The Author(s) (2019). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures
References
-
- Bani-Yaghoub M, Kendall SE, Moore DP, Bellum S, Cowling RA, Nikopoulos GN, et al. Insulin acts as a myogenic differentiation signal for neural stem cells with multilineage differentiation potential. Development 2004; 131: 4287–98. - PubMed
-
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006; 444: 756–60. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

