Neurobiological mechanisms of TENS-induced analgesia
- PMID: 30946953
- PMCID: PMC6547049
- DOI: 10.1016/j.neuroimage.2019.03.077
Neurobiological mechanisms of TENS-induced analgesia
Abstract
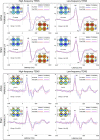
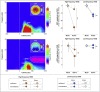
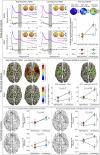
Pain inhibition by additional somatosensory input is the rationale for the widespread use of Transcutaneous Electrical Nerve Stimulation (TENS) to relieve pain. Two main types of TENS produce analgesia in animal models: high-frequency (∼50-100 Hz) and low-intensity 'conventional' TENS, and low-frequency (∼2-4 Hz) and high-intensity 'acupuncture-like' TENS. However, TENS efficacy in human participants is debated, raising the question of whether the analgesic mechanisms identified in animal models are valid in humans. Here, we used a sham-controlled experimental design to clarify the efficacy and the neurobiological effects of 'conventional' and 'acupuncture-like' TENS in 80 human volunteers. To test the analgesic effect of TENS we recorded the perceptual and brain responses elicited by radiant heat laser pulses that activate selectively Aδ and C cutaneous nociceptors. To test whether TENS has a long-lasting effect on brain state we recorded spontaneous electrocortical oscillations. The analgesic effect of 'conventional' TENS was maximal when nociceptive stimuli were delivered homotopically, to the same hand that received the TENS. In contrast, 'acupuncture-like' TENS produced a spatially-diffuse analgesic effect, coupled with long-lasting changes both in the state of the primary sensorimotor cortex (S1/M1) and in the functional connectivity between S1/M1 and the medial prefrontal cortex, a core region in the descending pain inhibitory system. These results demonstrate that 'conventional' and 'acupuncture-like' TENS have different analgesic effects, which are mediated by different neurobiological mechanisms.
Keywords: Analgesia; Electroencephalography (EEG); Human; Pain; Resting state; Transcutaneous electrical nerve stimulation (TENS).
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Supraspinal neural mechanisms of the analgesic effect produced by transcutaneous electrical nerve stimulation.Brain Struct Funct. 2021 Jan;226(1):151-162. doi: 10.1007/s00429-020-02173-9. Epub 2020 Nov 24. Brain Struct Funct. 2021. PMID: 33236208
-
An investigation of the development of analgesic tolerance to TENS in humans.Pain. 2011 Feb;152(2):335-342. doi: 10.1016/j.pain.2010.10.040. Epub 2010 Dec 8. Pain. 2011. PMID: 21144659 Free PMC article. Clinical Trial.
-
Conventional and acupuncture-like transcutaneous electrical nerve stimulation excite similar afferent fibers.Arch Phys Med Rehabil. 1993 Jan;74(1):54-60. Arch Phys Med Rehabil. 1993. PMID: 8420521
-
[Mechanisms and applications of transcutaneous electrical nerve stimulation in analgesia].Sheng Li Xue Bao. 2017 Jun 25;69(3):325-334. Sheng Li Xue Bao. 2017. PMID: 28638926 Review. Chinese.
-
Complementary and alternative therapies for post-caesarean pain.Cochrane Database Syst Rev. 2020 Sep 1;9(9):CD011216. doi: 10.1002/14651858.CD011216.pub2. Cochrane Database Syst Rev. 2020. PMID: 32871021 Free PMC article.
Cited by
-
Extensive sensorimotor training enhances nociceptive cortical responses in healthy individuals.Eur J Pain. 2023 Feb;27(2):257-277. doi: 10.1002/ejp.2057. Epub 2022 Dec 1. Eur J Pain. 2023. PMID: 36394423 Free PMC article.
-
Peripheral Electrical Stimulation Modulates Cortical Beta-Band Activity.Front Neurosci. 2021 Mar 25;15:632234. doi: 10.3389/fnins.2021.632234. eCollection 2021. Front Neurosci. 2021. PMID: 33867919 Free PMC article.
-
Primary Dysmenorrhea: Pathophysiology, Diagnosis, and Treatment Updates.Korean J Fam Med. 2022 Mar;43(2):101-108. doi: 10.4082/kjfm.21.0103. Epub 2022 Mar 17. Korean J Fam Med. 2022. PMID: 35320895 Free PMC article.
-
The Effects of Acupoint Stimulation Combined With Transcutaneous Electrical Nerve Stimulation on Labor Pain: Protocol for a Stepped Wedge Cluster Randomized Controlled Trial.JMIR Res Protoc. 2025 May 26;14:e63050. doi: 10.2196/63050. JMIR Res Protoc. 2025. PMID: 40418802 Free PMC article.
-
Effects and mechanisms of acupuncture analgesia mediated by afferent nerves in acupoint microenvironments.Front Neurosci. 2024 Feb 7;17:1239839. doi: 10.3389/fnins.2023.1239839. eCollection 2023. Front Neurosci. 2024. PMID: 38384495 Free PMC article. Review.
References
-
- Astolfi L., Cincotti F., Mattia D., Babiloni C., Carducci F., Basilisco A., Rossini P.M., Salinari S., Ding L., Ni Y., He B., Babiloni F. Assessing cortical functional connectivity by linear inverse estimation and directed transfer function: simulations and application to real data. Clin. Neurophysiol. 2005;116:920–932. - PubMed
-
- Babiloni C., Brancucci A., Del Percio C., Capotosto P., Arendt-Nielsen L., Chen A.C., Rossini P.M. Anticipatory electroencephalography alpha rhythm predicts subjective perception of pain intensity. J. Pain. 2006;7:709–717. - PubMed
-
- Babiloni F., Cincotti F., Babiloni C., Carducci F., Mattia D., Astolfi L., Basilisco A., Rossini P.M., Ding L., Ni Y., Cheng J., Christine K., Sweeney J., He B. Estimation of the cortical functional connectivity with the multimodal integration of high-resolution EEG and fMRI data by directed transfer function. Neuroimage. 2005;24:118–131. - PubMed
-
- Baeumler P.I., Fleckenstein J., Benedikt F., Bader J., Irnich D. Acupuncture-induced changes of pressure pain threshold are mediated by segmental inhibition--a randomized controlled trial. Pain. 2015;156:2245–2255. - PubMed
-
- Barlas P., Ting S.L., Chesterton L.S., Jones P.W., Sim J. Effects of intensity of electroacupuncture upon experimental pain in healthy human volunteers: a randomized, double-blind, placebo-controlled study. Pain. 2006;122:81–89. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

