Associations between baseline amyloid, sex, and APOE on subsequent tau accumulation in cerebrospinal fluid
- PMID: 30947113
- PMCID: PMC6545139
- DOI: 10.1016/j.neurobiolaging.2019.02.019
Associations between baseline amyloid, sex, and APOE on subsequent tau accumulation in cerebrospinal fluid
Abstract
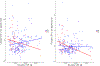
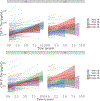
We investigated the effect of baseline Aβ, sex, and APOE on longitudinal tau accumulation in cerebrospinal fluid (CSF) in clinically normal older adults. Two hundred thirty-nine participants (aged 56-89 years, clinical dementia rating = 0) underwent serial CSF collection for Aβ1-42, total-tau (t-tau) and phospho-tau181P (p-tau). We used preprocessed data from fully automated Roche Elecsys immunoassays. A series of linear regressions were used to examine cross-sectional effects of Aβ1-42, sex, and APOEε4 on baseline CSF tau and linear mixed models for longitudinal changes in CSF tau. Cross-sectionally, CSF t-tau and p-tau were associated with abnormal Aβ1-42 and APOEε4 but not with sex. Longitudinally, low baseline CSF Aβ1-42 levels, but not APOEε4 or sex, predicted faster p-tau accumulation. The relationship between baseline CSF Aβ1-42 and tau accumulation was strongest in APOEε4 carriers, and particularly female carriers, relative to other groups. The current findings support an association between baseline CSF Aβ1-42 and changes in CSF tau. Elevated risk in females, apparent only in carriers, reinforces findings of sex-related vulnerability in those with genetic predisposition for Alzheimer's disease.
Keywords: APOE; Alzheimer's disease; Amyloid; Cerebrospinal fluid; Sex; tau.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures

References
-
- Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA, 2005. Sex differences in the clinical manifestations of Alzheimer disease pathology. Archives of general psychiatry 62(6), 685–691. - PubMed
-
- Bittner T, Zetterberg H, Teunissen CE, Ostlund RE, Militello M, Andreasson U, Hubeek I, Gibson D, Chu DC, Eichenlaub U, Heiss P, Kobold U, Leinenbach A, Madin K, Manuilova E, Rabe C, Blennow K, 2016. Technical performance of a novel, fully automated electrochemiluminescence immunoassay for the quantitation of β-amyloid (1–42) in human cerebrospinal fluid. Alzheimer’s & Dementia 12(5), 517–526. - PubMed
-
- Blennow K, Wallin A, Agren H, Spenger C, Siegfried J, Vanmechelen E, 1995. Tau protein in cerebrospinal fluid: a biochemical marker for axonal degeneration in Alzheimer disease? Mol Chem Neuropathol 26(3), 231–245. - PubMed
-
- Brier MR, Gordon B, Friedrichsen K, McCarthy J, Stern A, Christensen J, Owen C, Aldea P, Su Y, Hassenstab J, Cairns NJ, Holtzman DM, Fagan AM, Morris JC, Benzinger TLS, Ances BM, 2016. Tau and Aβ imaging, CSF measures, and cognition in Alzheimer’s disease. Science Translational Medicine 8(338), 338ra366–338ra366. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K24 AG035007/AG/NIA NIH HHS/United States
- R01 AG037497/AG/NIA NIH HHS/United States
- S10 RR021110/RR/NCRR NIH HHS/United States
- R00 AG061238/AG/NIA NIH HHS/United States
- R01 AG027435/AG/NIA NIH HHS/United States
- U19 AG024904/AG/NIA NIH HHS/United States
- R21 AG038994/AG/NIA NIH HHS/United States
- P50 AG005134/AG/NIA NIH HHS/United States
- K99 AG061238/AG/NIA NIH HHS/United States
- K23 AG049087/AG/NIA NIH HHS/United States
- R01 AG034556/AG/NIA NIH HHS/United States
- R01 EB014894/EB/NIBIB NIH HHS/United States
- R01 AG026484/AG/NIA NIH HHS/United States
- U01 AG032438/AG/NIA NIH HHS/United States
- P30 AG062421/AG/NIA NIH HHS/United States
- P01 AG036694/AG/NIA NIH HHS/United States
- U01 AG024904/AG/NIA NIH HHS/United States
- U19 AG010483/AG/NIA NIH HHS/United States
- K01 AG051718/AG/NIA NIH HHS/United States
- S10 RR023043/RR/NCRR NIH HHS/United States
- P41 EB015896/EB/NIBIB NIH HHS/United States
- S10 RR023401/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

