Cx32 exerts anti-apoptotic and pro-tumor effects via the epidermal growth factor receptor pathway in hepatocellular carcinoma
- PMID: 30947731
- PMCID: PMC6449973
- DOI: 10.1186/s13046-019-1142-y
Cx32 exerts anti-apoptotic and pro-tumor effects via the epidermal growth factor receptor pathway in hepatocellular carcinoma
Abstract
Background: Abnormal expression or distribution of connexin 32 (Cx32) is associated with hepatocarcinogenesis, but the role of Cx32 and the underlying mechanisms are still unclear.
Methods: The expression level of Cx32 in 96 hepatocellular carcinoma (HCC) specimens was determined using western blotting and immunohistochemistry. The correlation between Cx32 expression and clinicopathological parameters was analyzed. The cell apoptosis rate was examined using flow cytometry and western blotting. The role of Cx32 in the Src kinase and epidermal growth factor receptor (EGFR) signaling pathways was measured by quantitative real-time PCR, western blotting and coimmunoprecipitation (CO-IP). The effect of Cx32 overexpression on the streptonigrin (SN)-induced tumor growth suppression and apoptosis was assessed in nude mice.
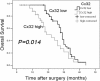
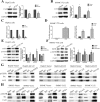
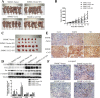
Results: Our study showed that overexpressed Cx32 accumulated in the cytoplasm and that Cx32-containing gap junctions (GJs) were nearly absent in HCC specimens. Upregulated Cx32 expression was highly correlated with advanced tumor-node-metastasis (TNM) stage and poor tumor differentiation and was an independent predictive marker for poor prognosis in HCC. Overexpression of Cx32 significantly inhibited SN-induced apoptosis by activating the EGFR signaling pathway in vitro and in vivo. Moreover, the expression levels of Cx32 and EGFR were positively correlated in HCC specimens. The CO-IP experiments demonstrated that Cx32 could bind to Src kinase, and the western blotting results revealed that Cx32 increased the levels of EGFR and p-EGFR by upregulating Src expression.
Conclusion: The present study demonstrated that overexpressed and internalized Cx32 was associated with advanced TNM stage and poor tumor differentiation and predicted poor prognosis in HCC. Cx32 facilitated HCC progression by blocking chemotherapy-induced apoptosis in vitro and in vivo via interacting with Src and thus promoting the phosphorylation of EGFR, subsequently activating the EGFR signaling pathway. Cx32 may be a potential biomarker and a new therapeutic target for HCC.
Keywords: Apoptosis; Connexin32; EGFR; Hepatocellular carcinoma; Nonjunctional function; Src.
Conflict of interest statement
Ethics approval and consent to participate
Ethical approval (K-201354)) of clinical data and tissues was obtained from the Research Committee of Ethics in the Affiliated Cancer Hospital of Xinjiang Medical University. Written informed consent form for the experimental studies was obtained from the patients or their guardians. All the animal experimental procedures were approved by the Institutional Animal Care and Use Committee of Sun Yat-sen University.
Consent for publication
All authors agree for publication.
Competing interests
All authors read and approved the final version of the manuscript, and the authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

