Sequential organ failure assessment score is an excellent operationalization of disease severity of adult patients with hospitalized community acquired pneumonia - results from the prospective observational PROGRESS study
- PMID: 30947753
- PMCID: PMC6450002
- DOI: 10.1186/s13054-019-2316-x
Sequential organ failure assessment score is an excellent operationalization of disease severity of adult patients with hospitalized community acquired pneumonia - results from the prospective observational PROGRESS study
Abstract
Background: CAP (Community acquired pneumonia) is frequent, with a high mortality rate and a high burden on health care systems. Development of predictive biomarkers, new therapeutic concepts, and epidemiologic research require a valid, reproducible, and quantitative measure describing CAP severity.
Methods: Using time series data of 1532 patients enrolled in the PROGRESS study, we compared putative measures of CAP severity for their utility as an operationalization. Comparison was based on ability to correctly identify patients with an objectively severe state of disease (death or need for intensive care with at least one of the following: substantial respiratory support, treatment with catecholamines, or dialysis). We considered IDSA/ATS minor criteria, CRB-65, CURB-65, Halm criteria, qSOFA, PSI, SCAP, SIRS-Score, SMART-COP, and SOFA.
Results: SOFA significantly outperformed other scores in correctly identifying a severe state of disease at the day of enrollment (AUC = 0.948), mainly caused by higher discriminative power at higher score values. Runners-up were the sum of IDSA/ATS minor criteria (AUC = 0.916) and SCAP (AUC = 0.868). SOFA performed similarly well on subsequent study days (all AUC > 0.9) and across age groups. In univariate and multivariate analysis, age, sex, and pack-years significantly contributed to higher SOFA values whereas antibiosis before hospitalization predicted lower SOFA.
Conclusions: SOFA score can serve as an excellent operationalization of CAP severity and is proposed as endpoint for biomarker and therapeutic studies.
Trial registration: clinicaltrials.gov NCT02782013 , May 25, 2016, retrospectively registered.
Keywords: Biomarker; Clinical epidemiology; Infectious disease; Lung disease; Prospective clinical study; Severity score.
Conflict of interest statement
Ethics approval and consent to participate
In this manuscript, data from the PROGRESS study (clinicaltrials.gov: NCT02782013) were used. PROGRESS was approved by the ethics committee of the University of Jena (2403–10/08) and by locally responsible ethics committees of each study center. All participants or their legal guardians gave written informed consent for participation in the study. Requirements of the Declaration of Helsinki [22] and the ICH-GCP guideline [23] were met.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

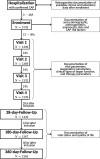

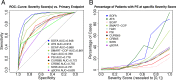

References
-
- WHO 2016. WHO report on global burden of disease 2000–2012, update 2014. http://www.who.int/healthinfo/global_burden_disease/en/. Accessed 9 Feb 2016.
-
- AQUA - Institut für angewandte Qualitätsförderung und Forschung im Gesundheitswesen GmbH. PNEU – PNEU - Ambulant erworbene Pneumonie. 2015. https://www.sqg.de/downloads/Bundesauswertungen/2014/bu_Gesamt_PNEU_2014.... Accessed 9 Feb 2016.
Publication types
MeSH terms
Associated data
Grants and funding
- 01KI07110/Bundesministerium für Bildung und Forschung/International
- 01KI07111/Bundesministerium für Bildung und Forschung/International
- 01KI07113/Bundesministerium für Bildung und Forschung/International
- 01KI07114/Bundesministerium für Bildung und Forschung/International
- 01KI1010I/Bundesministerium für Bildung und Forschung/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

