Targeted deletion of Tcf7l2 in adipocytes promotes adipocyte hypertrophy and impaired glucose metabolism
- PMID: 30948248
- PMCID: PMC6531814
- DOI: 10.1016/j.molmet.2019.03.003
Targeted deletion of Tcf7l2 in adipocytes promotes adipocyte hypertrophy and impaired glucose metabolism
Abstract
Objective: Activation of the Wnt-signaling pathway is known to inhibit differentiation in adipocytes. However, there is a gap in our understanding of the transcriptional network regulated by components of the Wnt-signaling pathway during adipogenesis and in adipocytes during postnatal life. The key intracellular effectors of the Wnt-signaling pathway occur through TCF transcription factors such as TCF7L2 (transcription factor-7-like 2). Several genetic variants in proximity to TCF7L2 have been linked to type 2 diabetes through genome-wide association studies in various human populations. Our work aims to functionally characterize the adipocyte specific gene program regulated by TCF7L2 and understand how this program regulates metabolism.
Methods: We generated Tcf7l2F/F mice and assessed TCF7L2 function in isolated adipocytes and adipose specific knockout mice. ChIP-sequencing and RNA-sequencing was performed on the isolated adipocytes with control and TCF7L2 knockout cells. Adipose specific TCF7L2 knockout mice were challenged with high fat diet and assessed for body weight, glucose tolerance, and lipolysis.
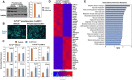
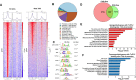
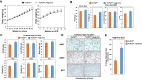
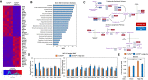
Results: Here we report that TCF7L2 regulates adipocyte size, endocrine function, and glucose metabolism. Tcf7l2 is highly expressed in white adipose tissue, and its expression is suppressed in genetic and diet-induced models of obesity. Genome-wide distribution of TCF7L2 binding and gene expression analysis in adipocytes suggests that TCF7L2 directly regulates genes implicated in cellular metabolism and cell cycle control. When challenged with a high-fat diet, conditional deletion of TCF7L2 in adipocytes led to impaired glucose tolerance, impaired insulin sensitivity, promoted weight gain, and increased adipose tissue mass. This was accompanied by reduced expression of triglyceride hydrolase, reduced fasting-induced free fatty acid release, and adipocyte hypertrophy in subcutaneous adipose tissue.
Conclusions: Together our studies support that TCF7L2 is a central transcriptional regulator of the adipocyte metabolic program by directly regulating the expression of genes involved in lipid and glucose metabolism.
Keywords: Adipose tissue; Diabetes; Lipolysis; Obesity; Wnt signaling.
Copyright © 2019. Published by Elsevier GmbH.
Figures




References
-
- Morin P.J., Sparks A.B., Korinek V., Barker N., Clevers H., Vogelstein B. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275(5307):1787–1790. - PubMed
-
- Nusse R., Varmus H.E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31(1):99–109. - PubMed
-
- Behrens J., von Kries J.P., Kuhl M., Bruhn L., Wedlich D., Grosschedl R. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382(6592):638–642. - PubMed
-
- Li V.S., Ng S.S., Boersema P.J., Low T.Y., Karthaus W.R., Gerlach J.P. Wnt signaling through inhibition of beta-catenin degradation in an intact Axin1 complex. Cell. 2012;149(6):1245–1256. - PubMed
-
- Cavallo R.A., Cox R.T., Moline M.M., Roose J., Polevoy G.A., Clevers H. Drosophila Tcf and Groucho interact to repress Wingless signalling activity. Nature. 1998;395(6702):604–608. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

