Thirst regulates motivated behavior through modulation of brainwide neural population dynamics
- PMID: 30948440
- PMCID: PMC6711472
- DOI: 10.1126/science.aav3932
Thirst regulates motivated behavior through modulation of brainwide neural population dynamics
Abstract
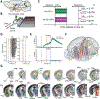
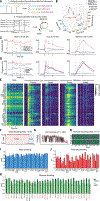
Physiological needs produce motivational drives, such as thirst and hunger, that regulate behaviors essential to survival. Hypothalamic neurons sense these needs and must coordinate relevant brainwide neuronal activity to produce the appropriate behavior. We studied dynamics from ~24,000 neurons in 34 brain regions during thirst-motivated choice behavior in 21 mice as they consumed water and became sated. Water-predicting sensory cues elicited activity that rapidly spread throughout the brain of thirsty animals. These dynamics were gated by a brainwide mode of population activity that encoded motivational state. After satiation, focal optogenetic activation of hypothalamic thirst-sensing neurons returned global activity to the pre-satiation state. Thus, motivational states specify initial conditions that determine how a brainwide dynamical system transforms sensory input into behavioral output.
Copyright © 2019, American Association for the Advancement of Science.
Figures




Comment in
-
Parsing signal and noise in the brain.Science. 2019 Apr 19;364(6437):236-237. doi: 10.1126/science.aax1512. Epub 2019 Apr 18. Science. 2019. PMID: 31000652 No abstract available.
References
-
- Hull C, Principles of Behavior: An Introduction to Behavior Theory (Appleton-Century, 1943).
-
- Bolles RC, Theory of Motivation (Harper & Row, 1967).
-
- Toates F, Motivational Systems (Cambridge Univ. Press, 1986).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

