Immunosuppressive therapy for pediatric aplastic anemia: a North American Pediatric Aplastic Anemia Consortium study
- PMID: 30948484
- PMCID: PMC6886407
- DOI: 10.3324/haematol.2018.206540
Immunosuppressive therapy for pediatric aplastic anemia: a North American Pediatric Aplastic Anemia Consortium study
Abstract
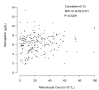
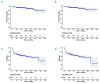
Quality of response to immunosuppressive therapy and long-term outcomes for pediatric severe aplastic anemia remain incompletely characterized. Contemporary evidence to inform treatment of relapsed or refractory severe aplastic anemia for pediatric patients is also limited. The clinical features and outcomes for 314 children treated from 2002 to 2014 with immunosuppressive therapy for acquired severe aplastic anemia were analyzed retrospectively from 25 institutions in the North American Pediatric Aplastic Anemia Consortium. The majority of subjects (n=264) received horse anti-thymocyte globulin (hATG) plus cyclosporine (CyA) with a median 61 months follow up. Following hATG/CyA, 71.2% (95%CI: 65.3,76.6) achieved an objective response. In contrast to adult studies, the quality of response achieved in pediatric patients was high, with 59.8% (95%CI: 53.7,65.8) complete response and 68.2% (95%CI: 62.2,73.8) achieving at least a very good partial response with a platelet count ≥50×109L. At five years post-hATG/CyA, overall survival was 93% (95%CI: 89,96), but event-free survival without subsequent treatment was only 64% (95%CI: 57,69) without a plateau. Twelve of 171 evaluable patients (7%) acquired clonal abnormalities after diagnosis after a median 25.2 months (range: 4.3-71 months) post treatment. Myelodysplastic syndrome or leukemia developed in 6 of 314 (1.9%). For relapsed/refractory disease, treatment with a hematopoietic stem cell transplant had a superior event-free survival compared to second immunosuppressive therapy treatment in a multivariate analysis (HR=0.19, 95%CI: 0.08,0.47; P=0.0003). This study highlights the need for improved therapies to achieve sustained high-quality remission for children with severe aplastic anemia.
Copyright© 2019 Ferrata Storti Foundation.
Figures


Comment in
-
Pediatric aplastic anemia treatment patterns and responses; power in the numbers.Haematologica. 2019 Oct;104(10):1909-1912. doi: 10.3324/haematol.2019.225870. Haematologica. 2019. PMID: 31575670 Free PMC article. No abstract available.
References
-
- Kojima S, Horibe K, Inaba J, et al. Long-term outcome of acquired aplastic anaemia in children: comparison between immunosuppressive therapy and bone marrow transplantation. Br J Haematol. 2000; 111(1):321-328. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

