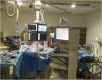
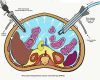
Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC): Initial Experience from Indian Centers and a Review of Literature
- PMID: 30948867
- PMCID: PMC6414563
- DOI: 10.1007/s13193-018-0771-5
Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC): Initial Experience from Indian Centers and a Review of Literature
Abstract
Cytoreductive surgery and HIPEC is a therapeutic option that benefits only selected patients with peritoneal metastases (PM). New treatments like pressurized intraperitoneal aerosol chemotherapy (PIPAC) have been developed to overcome some limitations of intraperitoneal chemotherapy and treat patients who are not eligible for a curative approach. The safety and feasibility of the procedure in the first few Indian patients treated with PIPAC, and the technique and the set-up required for PIPAC are described here. From May 2017 to August 2017, data was collected prospectively for all patients undergoing PIPAC at three Indian centers. The patients' characteristic, operative findings, and perioperative outcomes were recorded. Seventeen procedures were performed in 16 patients with peritoneal metastases from various primary sites using standard drug regimens developed for the procedure. The median hospital stay was 1 day, minor and major complications were seen in two patients each (11.7%), and there was one post-operative death. Of the six patients who completed at least 6 weeks of follow-up, there was disease progression in two, unrelated problems in two patients, and a second procedure was performed in one patient. One patient underwent subsequent CRS and HIPEC. Our results show the feasibility and safety of PIPAC in Indian patients with a low morbidity and mortality and short hospital stay. While clinical trials will determine its role in addition to systemic chemotherapy, it can be used in patients who have progressed on one or more lines of systemic chemotherapy and those who have chemotherapy-resistant ascites.
Keywords: Indian experience; PIPAC; PIPAC review; Peritoneal metastases.
Figures
References
-
- Glehen O, Elias D, Gilly F-N. Carcinoses Péritonéales D’Origine Digestive et Primitive: Rapport du 110ème Congrès de L’Association Française de Chirurgie—Monographie de L’Association française de Chirurgie. Arnette: Rueil-Malmaison, France; 2008. pp. 101–151.
-
- Franko J, Shi Q, Meyers JP, et al. (2016) Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol; published online Oct 12. 10.1016/S1470-2045(16)30500-9 - PubMed
-
- Grass F1, Vuagniaux A1, Teixeira-Farinha H1, Lehmann K2, Demartines N1, Hübner M1 (2017) Systematic review of pressurized intraperitoneal aerosol chemotherapy for the treatment of advanced peritoneal carcinomatosis. Br J Surg 104(6):669–678. 10.1002/bjs.10521 - PubMed
-
- Solass W, Kerb R, Mürdter T, Giger-Pabst U, Strumberg D, Tempfer C, Zieren J, Schwab M, Reymond MA. Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: first evidence for efficacy. Ann Surg Oncol. 2014;21(2):553–559. doi: 10.1245/s10434-013-3213-1. - DOI - PMC - PubMed
-
- https://evs.nci.nih.gov/.../CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8...4.03. Common Terminology Criteria for. Adverse Events (CTCAE). Version 4.0. Published: May 28, 2009 (v4.03: June 14, 2010)
LinkOut - more resources
Full Text Sources