Brain Microglial Activation in Chronic Pain-Associated Affective Disorder
- PMID: 30949019
- PMCID: PMC6436078
- DOI: 10.3389/fnins.2019.00213
Brain Microglial Activation in Chronic Pain-Associated Affective Disorder
Abstract
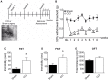
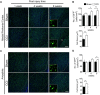
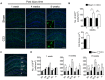
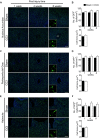
A growing body of evidence from both clinical and animal studies indicates that chronic neuropathic pain is associated with comorbid affective disorders. Spinal cord microglial activation is involved in nerve injury-induced pain hypersensitivity characterizing neuropathic pain. However, there is a lack of thorough assessments of microglial activation in the brain after nerve injury. In the present study, we characterized microglial activation in brain sub-regions of CX3CR1GFP/+ mice after chronic constriction injury (CCI) of the sciatic nerve, including observations at delayed time points when affective brain dysfunctions such as depressive-like behaviors typically develop. Mice manifested chronic mechanical hypersensitivity immediately after CCI and developed depressive-like behaviors 8 weeks post-injury. Concurrently, significant increases of soma size and microglial cell number were observed in the medial prefrontal cortex (mPFC), hippocampus, and amygdala 8 weeks post-injury. Transcripts of CD11b, and TNF-α, genes associated with microglial activation or depressive-like behaviors, are correspondingly upregulated in these brain areas. Our results demonstrate that microglia are activated in specific brain sub-regions after CCI at delayed time points and imply that brain microglial activation plays a role in chronic pain-associated affective disorders.
Keywords: TNF-α; brain microglia; chronic pain; depression; microglial activation.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials

