Neuron Names: A Gene- and Property-Based Name Format, With Special Reference to Cortical Neurons
- PMID: 30949034
- PMCID: PMC6437103
- DOI: 10.3389/fnana.2019.00025
Neuron Names: A Gene- and Property-Based Name Format, With Special Reference to Cortical Neurons
Abstract
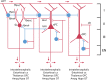
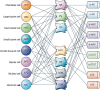
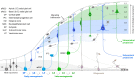
Precision in neuron names is increasingly needed. We are entering a new era in which classical anatomical criteria are only the beginning toward defining the identity of a neuron as carried in its name. New criteria include patterns of gene expression, membrane properties of channels and receptors, pharmacology of neurotransmitters and neuropeptides, physiological properties of impulse firing, and state-dependent variations in expression of characteristic genes and proteins. These gene and functional properties are increasingly defining neuron types and subtypes. Clarity will therefore be enhanced by conveying as much as possible the genes and properties in the neuron name. Using a tested format of parent-child relations for the region and subregion for naming a neuron, we show how the format can be extended so that these additional properties can become an explicit part of a neuron's identity and name, or archived in a linked properties database. Based on the mouse, examples are provided for neurons in several brain regions as proof of principle, with extension to the complexities of neuron names in the cerebral cortex. The format has dual advantages, of ensuring order in archiving the hundreds of neuron types across all brain regions, as well as facilitating investigation of a given neuron type or given gene or property in the context of all its properties. In particular, we show how the format is extensible to the variety of neuron types and subtypes being revealed by RNA-seq and optogenetics. As current research reveals increasingly complex properties, the proposed approach can facilitate a consensus that goes beyond traditional neuron types.
Keywords: axons; brain regions; dendrites; genomics; neuron classification; terminology.
Figures






References
-
- Ascoli G. A., Donohue D. E., Halavi M. (2007). NeuroMorpho.Org: a central resource for neuronal morphologies. J. Neurosci. 27 9247–9251. 10.1523/JNEUROSCI.2055-07.2007 - DOI - PMC - PubMed
-
- Ascoli G. A., Wheeler D. W. (2016). In search of a periodic table of the neurons: axonal-dendritic circuitry as the organizing principle - Patterns of axons and dendrites within distinct anatomical parcels provide the blueprint for circuit-based neuronal classification. Bioessays 38 969–976. 10.1002/bies.201600067 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

