The Central Role of Glucokinase in Glucose Homeostasis: A Perspective 50 Years After Demonstrating the Presence of the Enzyme in Islets of Langerhans
- PMID: 30949058
- PMCID: PMC6435959
- DOI: 10.3389/fphys.2019.00148
The Central Role of Glucokinase in Glucose Homeostasis: A Perspective 50 Years After Demonstrating the Presence of the Enzyme in Islets of Langerhans
Abstract
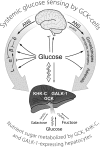
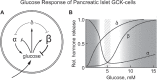
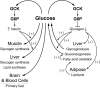
It is hypothesized that glucokinase (GCK) is the glucose sensor not only for regulation of insulin release by pancreatic β-cells, but also for the rest of the cells that contribute to glucose homeostasis in mammals. This includes other cells in endocrine pancreas (α- and δ-cells), adrenal gland, glucose sensitive neurons, entero-endocrine cells, and cells in the anterior pituitary. Glucose transport is by facilitated diffusion and is not rate limiting. Once inside, glucose is phosphorylated to glucose-6-phosphate by GCK in a reaction that is dependent on glucose throughout the physiological range of concentrations, is irreversible, and not product inhibited. High glycerol phosphate shuttle, pyruvate dehydrogenase, and pyruvate carboxylase activities, combined with low pentose-P shunt, lactate dehydrogenase, plasma membrane monocarboxylate transport, and glycogen synthase activities constrain glucose-6-phosphate to being metabolized through glycolysis. Under these conditions, glycolysis produces mostly pyruvate and little lactate. Pyruvate either enters the citric acid cycle through pyruvate dehydrogenase or is carboxylated by pyruvate carboxylase. Reducing equivalents from glycolysis enter oxidative phosphorylation through both the glycerol phosphate shuttle and citric acid cycle. Raising glucose concentration increases intramitochondrial [NADH]/[NAD+] and thereby the energy state ([ATP]/[ADP][Pi]), decreasing [Mg2+ADP] and [AMP]. [Mg2+ADP] acts through control of KATP channel conductance, whereas [AMP] acts through regulation of AMP-dependent protein kinase. Specific roles of different cell types are determined by the diverse molecular mechanisms used to couple energy state to cell specific responses. Having a common glucose sensor couples complementary regulatory mechanisms into a tightly regulated and stable glucose homeostatic network.
Keywords: counter regulatory hormones; diabetes; glucokinase; glucose homeostasis; metabolic regulation.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

