Complement Evasion: An Effective Strategy That Parasites Utilize to Survive in the Host
- PMID: 30949145
- PMCID: PMC6435963
- DOI: 10.3389/fmicb.2019.00532
Complement Evasion: An Effective Strategy That Parasites Utilize to Survive in the Host
Abstract
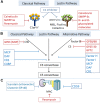
Parasitic infections induce host immune responses that eliminate the invading parasites. However, parasites have evolved to develop many strategies to evade host immune attacks and survive in a hostile environment. The complement system acts as the first line of immune defense to eliminate the invading parasites by forming the membrane attack complex (MAC) and promoting an inflammatory reaction on the surface of invading parasites. To date, the complement activation pathway has been precisely delineated; however, the manner in which parasites escape complement attack, as a survival strategy in the host, is not well understood. Increasing evidence has shown that parasites develop sophisticated strategies to escape complement-mediated killing, including (i) recruitment of host complement regulatory proteins on the surface of the parasites to inhibit complement activation; (ii) expression of orthologs of host RCA to inhibit complement activation; and (iii) expression of parasite-encoded proteins, specifically targeting different complement components, to inhibit complement function and formation of the MAC. In this review, we compiled information regarding parasitic abilities to escape host complement attack as a survival strategy in the hostile environment of the host and the mechanisms underlying complement evasion. Effective escape of host complement attack is a crucial step for the survival of parasites within the host. Therefore, those proteins expressed by parasites and involved in the regulation of the complement system have become important targets for the development of drugs and vaccines against parasitic infections.
Keywords: complement activation pathways; complement regulatory proteins; complement system; immune evasion; parasites.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

