The characterisation of interstitial lung disease multidisciplinary team meetings: a global study
- PMID: 30949489
- PMCID: PMC6441673
- DOI: 10.1183/23120541.00209-2018
The characterisation of interstitial lung disease multidisciplinary team meetings: a global study
Abstract
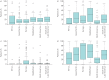
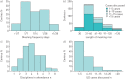
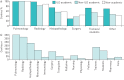
Multidisciplinary team (MDT) diagnosis of interstitial lung disease (ILD) has been proposed as a gold standard, but there are no formal recommendations for MDT process or composition and limited knowledge regarding prevalence in routine practice. We performed a systematic evaluation of ILD diagnostic practice across a range of healthcare settings around the world. Electronic questionnaires were distributed across all global regions via society and collaborators networks. Responses from 457 unique centres across 64 countries were included in the analysis. Of the 350 (76.6%) centres holding formal meetings, the majority held face-to-face MDT meetings (80%), for a minimum of 30 min (93%), and discussed diagnosis (96.9%) and patient management (94.9%) at the meetings. Compared with non-academic and academic non-ILD centres, ILD academic centres reported a higher ILD caseload, held more formal MDT meetings, and were more likely to include histopathology and rheumatology specialists in their diagnostic team. Of the centres holding MDT meetings, 5.5% routinely discussed all new cases at such meetings. An MDT approach to ILD diagnosis is consistently interpreted and widely implemented across a range of routine care settings around the world. This observation will inform future ILD diagnostic agreement studies and diagnostic pathway recommendations.
Conflict of interest statement
Conflict of interest: L. Richeldi reports personal fees for consulting activity from Sanofi-Aventis, ImmuneWorks, Celgene, Nitto, Bristol-Myers Squibb and Pliant Therapeutics, personal fees for membership of an advisory board from Roche, Fibrogen and Promedior, speaker fees from Shionogi, grants and personal fees for membership of a steering committee from Boehringer Ingelheim, and personal fees for editorial activity from DynaMed, outside the submitted work. Conflict of interest: N. Launders reports that the Respiratory Effectiveness Group received grants from Roche, Boehringer Ingelheim and Three Lakes Partners, and nonfinancial support from Veracyte, during the conduct of the study. Conflict of interest: F. Martinez reports personal fees, nonfinancial support and other support from Boehringer Ingelheim during the conduct of the study; personal fees and nonfinancial support from the American College of Chest Physicians, AstraZeneca, Boehringer Ingelheim, Continuing Education, ConCert, Genentech, GlaxoSmithKline, Miller Communications, the National Association for Continuing Education, Novartis, Pearl Pharmaceuticals, PeerView Communications, Prime Communications, the Puerto Rican Respiratory Society, Chiesi, Roche, Sunovion, Theravance, Potomac, the University of Alabama Birmingham, the Physicians Education Resource, the Canadian Respiratory Network and Teva, nonfinancial support from ProterrixBio, the Inova Fairfax Health System, Gilead, Nitto, Patara and Zambon, personal fees from Columbia University, Integritas, MD Magazine, Methodist Hospital Brooklyn, New York University, Unity, UpToDate, WebMD/MedScape, the Western Connecticut Health Network, Academic CME, PlatformIQ, the American Thoracic Society and Rockpointe, other support from Afferent/Merck, Biogen, Veracyte, Prometic, Bayer and Bridge Biotherapeutics, and grants from the NIH, Rare Disease Healthcare Communications and ProMedior, outside the submitted work. Conflict of interest: S.L.F. Walsh has nothing to disclose. Conflict of interest: J. Myers has nothing to disclose. Conflict of interest: B. Wang has nothing to disclose. Conflict of interest: M. Jones has nothing to disclose. Conflict of interest: A. Chisholm reports she worked for the Respiratory Effectiveness Group during the design and data capture stages of the study; she now works for Syneos Health. She has no conflicts of interest to declare in relation to this study. Conflict of interest: K.R. Flaherty reports grants and personal fees from Boehringer Ingelheim and Roche/Genentech, personal fees from Veracyte, Aeolus, Pharmakea, Sanofi-Genzyme and Fibrogen, outside the submitted work.
Figures

References
-
- Hutchinson J, Fogarty A, Hubbard R, et al. Global incidence and mortality of idiopathic pulmonary fibrosis: a systematic review. Eur Respir J 2015; 46: 795–806. - PubMed
-
- American Thoracic Society, European Respiratory Society American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2002; 165: 277–304. - PubMed
LinkOut - more resources
Full Text Sources
