Digital PCR improves the quantitation of DMR and the selection of CML candidates to TKIs discontinuation
- PMID: 30950237
- PMCID: PMC6536984
- DOI: 10.1002/cam4.2087
Digital PCR improves the quantitation of DMR and the selection of CML candidates to TKIs discontinuation
Abstract
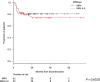
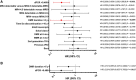
Treatment-free remission (TFR) by tyrosine kinase inhibitors (TKI) discontinuation in patients with deep molecular response (DMR) is a paramount goal in the current chronic myeloid leukemia (CML) therapeutic strategy. The best DMR level by real-time quantitative PCR (RT-qPCR) for TKI discontinuation is still a matter of debate. To compare the accuracy of digital PCR (dPCR) and RT-qPCR for BCR-ABL1 transcript levels detection, 142 CML patients were monitored for a median time of 24 months. Digital PCR detected BCR-ABL1 transcripts in the RT-qPCR undetectable cases. The dPCR analysis of the samples, grouped by the MR classes, revealed a significant difference between MR4.0 and MR4.5 (P = 0.0104) or MR5.0 (P = 0.0032). The clinical and hematological characteristics of the patients grouped according to DMR classes (MR4.0 vs MR4.5-5.0 ) were superimposable. Conversely, patients with dPCR values <0.468 BCR-ABL1 copies/µL (as we previously described) showed a longer DMR duration (P = 0.0220) and mainly belonged to MR4.5-5.0 (P = 0.0442) classes compared to patients with higher dPCR values. Among the 142 patients, 111 (78%) discontinued the TKI treatment; among the 111 patients, 24 (22%) lost the MR3.0 or MR4.0 . RT-qPCR was not able to discriminate patients with higher risk of MR loss after discontinuation (P = 0.8100). On the contrary, according to dPCR, 12/25 (48%) patients with BCR-ABL1 values ≥0.468 and 12/86 (14%) patients with BCR-ABL1 values <0.468 lost DMR in this cohort, respectively (P = 0.0003). Treatment-free remission of patients who discontinued TKI with a dPCR <0.468 was significantly higher compared to patients with dPCR ≥ 0.468 (TFR at 2 years 83% vs 52% P = 0.0017, respectively). In conclusion, dPCR resulted in an improved recognition of stable DMR and of candidates to TKI discontinuation.
Keywords: chronic myeloid leukemia; digital PCR (dPCR); minimal residual disease (MRD) monitoring; treatment-free remission (TFR); tyrosine kinase inhibitors (TKI) discontinuation.
© 2019 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

