How best to use photons
- PMID: 30950396
- PMCID: PMC6450062
- DOI: 10.1107/S2059798319003528
How best to use photons
Abstract
Strategies for collecting X-ray diffraction data have evolved alongside beamline hardware and detector developments. The traditional approaches for diffraction data collection have emphasised collecting data from noisy integrating detectors (i.e. film, image plates and CCD detectors). With fast pixel array detectors on stable beamlines, the limiting factor becomes the sample lifetime, and the question becomes one of how to expend the photons that your sample can diffract, i.e. as a smaller number of stronger measurements or a larger number of weaker data. This parameter space is explored via experiment and synthetic data treatment and advice is derived on how best to use the equipment on a modern beamline. Suggestions are also made on how to acquire data in a conservative manner if very little is known about the sample lifetime.
Keywords: data analysis; data collection; data processing; radiation damage.
open access.
Figures

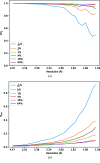
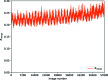
 to 64%, processed to a fixed resolution of 1.6 Å. Clearly the weakest of these data are suffering from poor precision in the intensity measurements, which rapidly improve as a greater dose is applied. There is, however, a point of diminishing returns between 1 and 16% where radiation damage becomes a greater factor in data quality than counting statistics.
to 64%, processed to a fixed resolution of 1.6 Å. Clearly the weakest of these data are suffering from poor precision in the intensity measurements, which rapidly improve as a greater dose is applied. There is, however, a point of diminishing returns between 1 and 16% where radiation damage becomes a greater factor in data quality than counting statistics.
 to 64%. Signs of radiation damage are clearly visible in the electron density in the last of these data sets. Of particular interest is the similarity in the maps (b)–(e): by eye there is very little difference in the maps despite the factor of 64 difference in transmission used.
to 64%. Signs of radiation damage are clearly visible in the electron density in the last of these data sets. Of particular interest is the similarity in the maps (b)–(e): by eye there is very little difference in the maps despite the factor of 64 difference in transmission used.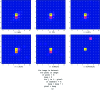

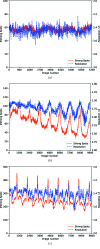
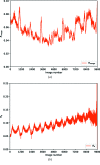
 (i.e. equivalent photon flux from 3.1 × 1011 to 3.9 × 1010 photons s−1) and the total rotation included (i.e. all 720°, first 360°, 180° and 90° of the data). Data sets included in each plot are in principle comparable, as the product of the rotation and transmission factor is constant.
(i.e. equivalent photon flux from 3.1 × 1011 to 3.9 × 1010 photons s−1) and the total rotation included (i.e. all 720°, first 360°, 180° and 90° of the data). Data sets included in each plot are in principle comparable, as the product of the rotation and transmission factor is constant.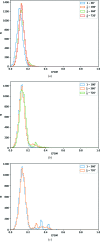
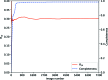
 original total photon count (a)
original total photon count (a)  (b) and
(b) and  (c).
(c).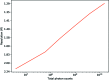





References
-
- Adams, P. D., Afonine, P. V., Bunkóczi, G., Chen, V. B., Davis, I. W., Echols, N., Headd, J. J., Hung, L.-W., Kapral, G. J., Grosse-Kunstleve, R. W., McCoy, A. J., Moriarty, N. W., Oeffner, R., Read, R. J., Richardson, D. C., Richardson, J. S., Terwilliger, T. C. & Zwart, P. H. (2010). Acta Cryst. D66, 213–221. - PMC - PubMed
-
- Diederichs, K. (2006). Acta Cryst. D62, 96–101. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

